UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
DC 20549
FORM
10-K
FOR
ANNUAL AND TRANSITION REPORTS
PURSUANT
TO SECTIONS 13 OR 15(d) OF
THE
SECURITIES
EXCHANGE ACT OF 1934
(Mark
One)
|
x
|
ANNUAL
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
fiscal year ended December 31,
2008
OR
|
o
|
TRANSITION
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
transition period from ______ to ______
Commission
file number 01-21617
THE
QUIGLEY CORPORATION
(Exact
Name of Registrant as Specified in Its Charter)
|
NEVADA
|
23-2577138
|
|
(State
or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S.
Employer Identification
No.)
|
|
KELLS BUILDING, 621
SHADY RETREAT ROAD, P.O. BOX 1349, DOYLESTOWN, PA
|
18901
|
|
(Address
of Principal Executive Offices)
|
(Zip
Code)
|
Registrant’s
telephone number, including area code (215)
345-0919
Securities
registered pursuant to Section 12(b) of the Act:
|
Title
of each class
|
Name
of each exchange on which registered
|
|
|
COMMON
STOCK, $.0005 PAR VALUE PER SHARE
|
NASDAQ
GLOBAL MARKET
|
|
|
COMMON
SHARE PURCHASE RIGHTS
|
NASDAQ
GLOBAL MARKET
|
Securities
registered pursuant to Section 12(g) of the Act: None
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities
Act. Yes o No x
Indicate
by check mark
if the registrant is not required to file reports pursuant to Section 13 or
Section 15(d) of the Act. Yes o No x
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes x No o
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not
be contained, to the best of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See
definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of
the Exchange Act. (Check one):
|
Large
accelerated filer o
|
Accelerated
filer o
|
|
Non-accelerated
filer o
|
Smaller
reporting company x
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Act). Yes o No x
The
aggregate market value of the
registrant’s common stock held by non-affiliates was $45,422,959 as of June 30,
2008, based on the closing price of the common stock on The NASDAQ Global
Market.
Number of
shares of each of the
registrant’s classes of securities outstanding on March 6, 2009:
Common
stock, $.0005 par value per share: 12,908,383.
Common
share purchase rights: 0
DOCUMENTS
INCORPORATED BY REFERENCE
Information
set forth in Part III of this report is incorporated by reference from the
registrant’s proxy statement for the 2009 annual meeting of
stockholders.
TABLE OF
CONTENTS
|
Part
I
|
||||
|
Page
|
||||
|
Item
|
1.
|
2
–13
|
||
|
1A.
|
13–
20
|
|||
|
1B.
|
20
|
|||
|
2.
|
20
|
|||
|
3.
|
21
– 23
|
|||
|
4.
|
23
|
|||
|
Part
II
|
5.
|
23
– 25
|
||
|
6.
|
25
|
|||
|
7.
|
26
– 32
|
|||
|
7A.
|
32
|
|||
|
8.
|
33
|
|||
|
9.
|
34
|
|||
|
9A
(T).
|
34
|
|||
|
9B.
|
35
|
|||
|
Part
III
|
10.
|
35
|
||
|
11.
|
35
|
|||
|
12.
|
35
|
|||
|
13.
|
35
|
|||
|
14.
|
35
|
|||
|
Part
IV
|
15.
|
36
|
||
|
Signatures
|
37
|
Forward-Looking
Statements
In
addition to historical information, this Report contains forward-looking
statements. These forward-looking statements are subject to certain risks and
uncertainties that could cause actual results to differ materially from those
reflected in these forward-looking statements. Factors that might cause such a
difference include, but are not limited to, management of growth, competition,
pricing pressures on the Company’s products, industry growth and general
economic conditions. Readers are cautioned not to place undue reliance on these
forward-looking statements, which reflect management’s opinions only as of the
date hereof. The Company undertakes no obligation to revise or publicly release
the results of any revision to these forward-looking statements.
Certain Risk
Factors
The
Quigley Corporation makes no representation that the United States Food and Drug
Administration (“FDA”) or any other regulatory agency will grant an
Investigational New Drug (“IND”) or take any other action to allow its
formulations to be studied or/and for any granted IND to be
marketed. Furthermore, no claim is made that potential medicine
discussed herein is safe, effective, or approved by the
FDA. Additionally, data that demonstrates activity or effectiveness
in animals or in vitro tests do not necessarily mean such formula test compound,
referenced herein, will be effective in humans. Safety and
effectiveness in humans will have to be demonstrated by means of adequate and
well controlled clinical studies before the clinical significance of the formula
test compound is known. Readers should carefully review the risk
factors described in other sections of the filing as well as in other documents
the Company files from time to time with the Securities and Exchange Commission
(“SEC”).
PART
I
ITEM
1. BUSINESS
Business
Development
The
Company, headquartered in Doylestown, Pennsylvania, is a leading manufacturer,
marketer and distributor of a diversified range of homeopathic and health
products which comprise the Cold Remedy and Contract Manufacturing
segments. The Company is also involved in the research and
development of potential natural base health products, including, but not
limited to, prescription medicines along with supplements and cosmeceuticals for
human and veterinary use, which comprise the Ethical Pharmaceutical
segment.
Cold-EezeÒ is one of the Company’s
key cold remedy OTC products whose benefits are derived from its proprietary
zinc formulation. The product’s effectiveness has been substantiated
in two double-blind clinical studies proving that Cold-EezeÒ reduces the duration and
severity of the common cold symptoms by nearly half. The Cold Remedy segment,
where Cold-EezeÒ is
represented, is reviewed regularly to realize any new consumer opportunities in
flavor, convenience and packaging to help improve market share for the
Cold-EezeÒ
product. Additionally, the Company is constantly active in
exploring and developing new products consistent with its brand image and
standard of proven consumer benefit.
Effective
October 1, 2004, the Company acquired substantially all of the assets of JoEl,
Inc., the previous manufacturer of the Cold-EezeÒ lozenge product assuring a
future manufacturing capability necessary to support the business of the Cold
Remedy segment. This manufacturing entity, now called Quigley
Manufacturing Inc. (“QMI”), a wholly-owned subsidiary of the Company operates
from two locations, Elizabethtown, PA, and Lebanon, PA. The location
at Lebanon manufacturers the Company’s Cold-Eeze lozenge product, and is
responsible for warehousing, shipping and such operational tasks for this
product and related cold remedy products. The Elizabethtown facility
produces a variety of hard and organic candy for sale to third party customers
in addition to performing contract manufacturing activities for non-related
entities. On February 2, 2009, the Company announced its intention to
close the Elizabethtown location of QMI and discontinue the hard candy business
resulting in the consolidation of manufacturing operations at the Lebanon
location. This consolidation will have no impact on the production or
distribution of the Cold-EezeÒ brand of cold remedy
products.
In
January 2001, the Company formed an Ethical Pharmaceutical segment, Quigley
Pharma Inc. (“Pharma”), that is under the
direction of its Executive Vice President and Chairman of its Medical Advisory
Committee. Pharma was formed for the purpose of developing potential
natural base health products, including, but not limited to, prescription
medicines along with supplements and cosmeceuticals for human and veterinary
use. Pharma is currently undergoing research
and development activity in compliance with
regulatory requirements. At this time, thirteen U.S. and twenty foreign patents
have been issued and assigned
to the Company resulting from research activity of Pharma. In certain
instances where a
critical mass of positive scientific data has been established for compounds
that the Company does not envision bringing to market, or is unable to fund
ongoing research, it may decide to sell or license its technology.
On
February 29, 2008, the Company sold Darius International Inc. (“Darius”) to
InnerLight Holdings, Inc., whose major shareholder is Mr. Kevin P. Brogan, the
then president of Darius. Darius marketed health and wellness
products through its wholly-owned subsidiary, Innerlight Inc. that constituted
the Health and Wellness segment of the Company. The terms of the sale
agreement included a cash purchase price of $1,000,000 by InnerLight Holdings,
Inc. for the stock of Darius and its subsidiaries without guarantees, warranties
or indemnifications. See discussion in Note 3 to Consolidated
Financial Statements.
Description of Business
Operations
Since its
inception, the Company has continued to conduct research and development into
various types of health-related supplements and homeopathic cold
remedies. Initially, the Company’s business was the marketing and
distribution of a line of nutritious health supplements (hereinafter
“Nutri-Bars”). During 1995, the Company reduced the marketing
emphasis of Nutri-Bars and commenced focusing its research and development and
marketing resources on the Company’s patented Cold-EezeÒ zinc gluconate glycine
cold relief products.
Prior to
the fourth quarter 1996, the Company had minimal revenues and as a result
suffered continued losses due to ongoing research and development and operating
expenses. However, 1997 resulted in significant revenue increases as a result of
the Company’s nationwide marketing campaign and the increased public awareness
through media public service announcements of the Cold-EezeÒ lozenge
product.
Since
June 1996, the Cold Remedy segment has concentrated its business operations on
the manufacturing, marketing and development of its proprietary Cold-EezeÒ cold-remedy lozenge
products and on development of various product extensions. These
products are based upon a proprietary zinc gluconate glycine formula, which has
been proven to reduce the duration and severity of common cold
symptoms. The Quigley Corporation acquired
worldwide manufacturing and distribution rights to this
formulation in 1992 and commenced national marketing in 1996. The
demand for the Company’s cold-remedy products is seasonal, where the third and
fourth quarters generally represent the largest sales volume. Prior
to October 1, 2004, the manufacture of the lozenge form of Cold-EezeÒ was
outsourced. Since that date, the lozenge form of Cold-EezeÒ has been manufactured by a
subsidiary of the Company, QMI. On February 2, 2009, the Company announced its
intention to close the Elizabethtown location of QMI and discontinue the hard
candy business resulting in the consolidation of manufacturing operations at the
Lebanon location. This consolidation will have no impact on the
production or distribution of the Cold-EezeÒ brand of cold remedy
products, which will continue to be produced and distributed from the Lebanon,
PA location.
Pharma is
currently involved in the lengthy process of conducting research and development
on certain of its patented formulations in compliance with FDA
regulations required for bringing prescriptions and botanical drugs to
market. The Company is in the initial stages of what may be a lengthy
process to develop these patent applications into potential commercial
products.
On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc., whose
major shareholder is Mr. Kevin P. Brogan, the then president of
Darius. Darius marketed health and wellness products through its
wholly-owned subsidiary, Innerlight Inc. that constituted the Health and
Wellness segment of the Company. The terms of the sale agreement
included a cash purchase price of $1,000,000 by InnerLight Holdings, Inc. for
the stock of Darius and its subsidiaries without guarantees, warranties or
indemnifications. See discussion in Note 3 to Consolidated Financial
Statements.
In 2008,
2007 and 2006, approximately 0% of the Company’s net sales, for all periods,
were related to international markets.
Financial
information regarding the Company’s operating segments is set forth in Item 8,
Notes to Financial Statements, Note 16 – Segment Information.
Products
Cold-Remedy
Products
In May
1992, the Company entered into an exclusive agreement for worldwide
representation, manufacturing, marketing of
Cold-EezeÒ products in
the United States. Cold-EezeÒ, a zinc gluconate glycine
formulation (ZIGG™), is an over-the-counter
consumer product used to reduce the duration and severity of the common cold and
is available in lozenge, sugar-free tablet and gum form. The Company
has substantiated the effectiveness of Cold-EezeÒ through a variety of
studies. A randomized double-blind placebo-controlled study,
conducted at Dartmouth College of Health Science, Hanover, New Hampshire,
concluded that the lozenge formulation treatment, initiated within 48 hours of
symptom onset, resulted in a significant reduction in the total duration of the
common cold.
On May 22, 1992, “Zinc and the Common
Cold, a Controlled Clinical Study,” was published in England in the
“Journal of International Medical Research,” Volume 20, Number 3, Pages
234-246. According to this publication, (a) flavorings used in other
Zinc lozenge products (citrate, tartrate, separate, orotate, picolinate,
mannitol or sorbitol) render the Zinc inactive and unavailable to the patient’s
nasal passages, mouth and throat where cold symptoms have to be treated, (b)
this patented formulation delivers approximately 93% of the active Zinc to the
mucosal surfaces and (c) the patient has the same sequence of symptoms as in the
absence of treatment but goes through the phases at an accelerated rate and with
reduced symptom severity.
On July
15, 1996, results of a new randomized double-blind placebo-controlled study on
the common cold, which commenced at the Cleveland Clinic Foundation
on October 3, 1994, were published. The study called “Zinc Gluconate Lozenges for Treating
the Common Cold” was completed and published in the Annals of Internal Medicine –
Vol. 125 No. 2. Using a 13.3mg lozenge (almost half the
strength of the lozenge used in the Dartmouth Study), the result still showed a
42% reduction in the duration of common cold symptoms.
In April
2002, the Company announced the statistical results of a retrospective clinical
adolescent study at the Heritage School facility in Provo, Utah that suggests
that Cold-EezeÒ is
also an effective means of preventing the common cold and statistically (a)
lessens the number of colds an individual suffers per year, reducing the median
from 1.5 to zero and (b) reduces the use of antibiotics for respiratory
illnesses from 39.3% to 3.0% when Cold-EezeÒ is administered as a first
line treatment approach to the common cold.
In April
2002, the Company was assigned a Patent Application which was filed with the
Patent Office of the United States Commerce Department for the use of
Cold-EezeÒ as a
prophylactic for cold prevention. The new patent application follows
the results of the adolescent study at the Heritage School
facility.
In May
2003, the Company announced the findings of a prospective study, conducted at
the Heritage School facility in Provo, Utah, in which 178 children, ages 12 to
18 years, were given Cold-EezeÒ lozenges both
symptomatically and prophylactically from October 5, 2001 to May 30, 2002. The
study found a 54% reduction in the most frequently observed cold duration. Those
subjects not receiving treatment most frequently experienced symptom duration of
11 days compared with 5 days when Cold-EezeÒ lozenges were
administered, a reduction of 6 days.
The
business of the Company is subject to federal and state laws and regulations
adopted for the health and safety of users of the Company’s products. Cold-Eeze®
is a homeopathic remedy that is subject to regulations by various federal, state
and local agencies, including the United States Food and Drug Administration
(“FDA”) and the Homeopathic Pharmacopoeia of the United States.
Contract
Manufacturing
From
October 1, 2004, QMI has continued to produce lozenge product along with
performing such operational tasks as warehousing and shipping the Company’s
Cold-EezeÒ
products. In addition to that function, QMI produces a variety of
hard and organic candy for sale to third party customers in addition to
performing contract manufacturing activities for non-related
entities. QMI is an FDA-approved facility.
On
February 2, 2009, the Company announced its intention to close the Elizabethtown
location of QMI and discontinue the hard candy business resulting in the
consolidation of manufacturing operations at the Lebanon
location. This consolidation will have no impact on the production or
distribution of the Cold-EezeÒ brand of cold remedy
products.
Ethical
Pharmaceutical
Pharma’s
current activity is the research and development of potential natural base
health products, including, but not limited to, prescription medicines along
with supplements and cosmeceuticals for human and veterinary
use. Research and development will focus on the identification,
isolation and direct use of active medicinal substances. One aspect
of Pharma’s research will focus on the potential synergistic benefits of
combining isolated active constituents and whole plant components. The Company
will search for new natural sources of medicinal substances from plants and
fungi from around the world while also investigating the use of traditional and
historic medicinals and therapeutics.
The
pre-clinical development, clinical trials, product manufacturing and marketing
of Pharma's
potential new products are subject to federal and state regulation in the United
States and other countries. Obtaining FDA regulatory approval for these
pharmaceutical products can require substantial resources and take several
years. The length of this process depends on the type, complexity and
novelty of the product and the nature of the disease or other indications to be
treated. If the Company cannot obtain regulatory approval of these new products
in a timely manner or if the patents are not granted or if the patents are
subsequently challenged, these possible events could have a material effect on
the business and financial condition of the Company. The strength of
the Company’s patent position may be important to its long-term
success. There can be no assurance that these patents and patent
applications will effectively protect the Company’s products from duplication by
others. Additionally, the operations of the Company contribute
to the current research and development expenditures of the Ethical
Pharmaceutical segment. In addition to the funding from
operations, the Company may in the short and long term raise capital through the
issuance of equity securities or secure other financing resources to support
such research. As research progresses on certain formulations,
expenditures of the Pharma segment will require substantial financial support
and would necessitate the consideration of other approaches such as, licensing
or partnership arrangements that meet the Company’s long term goals and
objectives. Ultimately, should internal working capital or internal
funding be insufficient, there is no guarantee that other financing resources
will become available, thereby deferring future growth and development of
certain formulations.
Patents
and chronological summary of QR formulations, which may or may not be areas of
current focus, are:
|
·
|
A
Patent (No. 6,555,573 B2) entitled “Method and Composition for the Topical
Treatment of Diabetic Neuropathy.” The patent extends through March 27,
2021.
|
|
·
|
A
Patent (No. 6,592,896 B2) entitled “Medicinal Composition and Method of
Using It” (for Treatment of Sialorrhea and other Disorders) for a product
to relieve sialorrhea (drooling) in patients suffering from Amyotrophic
Lateral Sclerosis (ALS), otherwise known as Lou Gehrig’s
Disease. The patent extends through August 5,
2021.
|
|
·
|
A
Patent (No. 6,596,313 B2) entitled “Nutritional Supplement and Method of
Using It” for a product to relieve sialorrhea (drooling) in patients
suffering from Amyotrophic Lateral Sclerosis (ALS), otherwise known as Lou
Gehrig’s Disease. The patent extends through April 14,
2022.
|
|
·
|
A
Patent (No. 6,753,325 B2) entitled “Composition and Method for Prevention,
Reduction and Treatment of Radiation Dermatitis,” a composition for
preventing, reducing or treating radiation dermatitis. The
patent extends through November 5,
2021.
|
|
·
|
A
Patent (No. 6,827,945 B2) entitled “Nutritional Supplements and Method of
Using Same” for a method for treating at least one symptom of
arthritis. The patent extends through April 22,
2023.
|
|
·
|
A
Patent (No. 7,083,813 B2) entitled “Methods for The Treatment of
Peripheral Neural and Vascular Ailments.” The patent extends
through August 4, 2023.
|
|
·
|
A
Patent (No. 7,166,435 B2) entitled “Compositions and Methods for Reducing
the Tranmissivity of Illnesses.” This patent will provide
additional protection to an existing composition patent (number
6,592,896), which the Company received in July 2003 and will support
on-going investigations and potential commercialization opportunities. The
Company will be continuing its studies to test the effects of the
referenced compound against avian flu and human influenza. The patent
extends through November 5, 2021.
|
|
·
|
A
Patent (No. 7,175,987 B2) entitled “Compositions and Methods for The
Treatment of Herpes.” The patent extends through November 5,
2021.
|
|
·
|
A
Patent (No. 7,396,546 B2) entitled “Anti-Microbial Compositions and
Methods of Using Same” The patent extends through August 6,
2021.
|
|
·
|
A
Patent (No. 7,399,783 B2) entitled “Methods for the Treatment of Scar
Tissue.” The patent extends through September 4,
2026.
|
|
·
|
A
Patent (No. 7,405,046 B2) entitled “Compositions and Methods for Treatment
of Rhinovirus.” The patent extends through August 6,
2021.
|
|
·
|
A
Patent (No. 7,410,659 B2) entitled “Methods for the Treatment of
Peripheral Neural and Vascular Ailments.” The patent extends
through November 6, 2022.
|
|
·
|
A
Patent (No. 7,435,725 B2) entitled “Oral Compositions and Methods for
Prevention, Reduction and Treatment of Radiation Injury.” The
patent extends through January 14,
2022.
|
|
·
|
A
Mexican Patent (No. 236311) entitled “Method and Composition for the
Treatment of Diabetic Neuropathy.” The patent extends through
December 18, 2020.
|
|
·
|
A
Mexican Patent (No. 259329) entitled “Nutritional Supplements and Methods
for Prevention, Reduction and Treatment of Radiation Injury” the patent
extends through April 30, 2022.
|
|
·
|
A
New Zealand Patent (No. 533439) entitled “Methods for The Treatment of
Peripheral Neural and Vascular Ailments.” The patent extends
through November 6, 2022.
|
|
·
|
A
New Zealand Patent (No. 526041) entitled “Method and Composition for the
Treatment of Diabetic Neuropathy.” The patent extends through December 18,
2021.
|
|
·
|
A
New Zealand Patent (No. 530187) entitled “Nutritional Supplements and
Methods of Using Same.” The patent extends through August 6,
2022.
|
|
·
|
A
New Zealand Patent (No. 537821) entitled “Anti-Microbial Compositions and
Methods of Using Same.” The patent extends through July 23,
2023.
|
|
·
|
A
New Zealand Patent (No. 532775) entitled “Topical Compositions and Methods
for Treatment of Adverse Effects of Ionizing Radiation,” The patent
extends through November 6, 2022.
|
|
·
|
An
Australian Patent (No. 2002231095) entitled “Method and Composition for
the Treatment of Diabetic Neuropathy.” The patent
extends through December 18, 2021.
|
|
·
|
An
Australian Patent (No. 2002352501) entitled “Method for The Treatment of
Peripheral Neural and Vascular Ailments.” The
patent extends through November 5,
2022.
|
|
·
|
An
Australian Patent (No. 2002232464) entitled “Nutritional Supplements and
Methods of Using Same.” The patent extends through
August 5, 2022.
|
|
·
|
An
Australian Patent (No. 2002365155) “Topical Compositions and Methods for
Treatment of Adverse Effects of Ionizing Radiation,” the patent extends
through November 5, 2022.
|
|
·
|
An
Australian Patent (No. 2002309615) “Nutritional Supplements and Methods
for Prevention, Reduction and Treatment of Radiation Injury” the patent
extends through April 30, 2022.
|
|
·
|
A
South African Patent (No. 2003/4247) entitled “Methods and Composition for
the Treatment of Diabetic Neuropathy.” The patent
extends through December 18, 2021.
|
|
·
|
A
South African Patent (No. 2004/3364) “Nutritional Supplements and Methods
for Prevention, Reduction and Treatment of Radiation Injury” the patent
extends through May 1, 2022.
|
|
·
|
A
South African Patent (No. 2003/9802) entitled “Nutritional Supplements and
Methods of Using Same” for a method for treating at least one symptom of
arthritis. The patent extends through August 5,
2022.
|
|
·
|
A
South African Patent (No. 2004/4614) entitled “Methods for The Treatment
of Peripheral Neural and Vascular Ailments.” The patent extends
through November 5, 2022.
|
|
·
|
A
South African Patent (No. 2005/0517) entitled “Anti-Microbial Compositions
& Methods for Using Same,” the patent extends through July 23,
2023.
|
|
·
|
A
South African Patent (No. 2004/3365) “Topical Compositions and Methods for
Treatment of Adverse Effects of Ionizing Radiation,” the patent extends
through November 5, 2022.
|
|
·
|
An
Israeli Patent (No. 159357) entitled “Nutritional Supplements and Methods
of Using Same,” the patent extends through August 6,
2022.
|
|
·
|
An
Indian Patent (No. 00004/MUMP/2004) entitled “A Nutritional
Supplement.” The patent extends through August 6,
2022.
|
QR-333 – In April 2002, the
Company initiated a Proof of Concept Study in France for treatment of diabetic
neuropathy, which was concluded in 2003. In April 2003, the Company
announced that an independently monitored analysis of the
Proof of Concept Study concluded that subjects using
this formulation had 67% of their
symptoms improve, suggesting efficacy. In March 2004, the Company
announced that it had completed its first meeting at the FDA prior to submitting
the Company’s IND application for the relief of symptoms of diabetic symmetrical
peripheral neuropathy. The FDA’s pre-IND meeting programs are
designed to provide sponsors with advance guidance and input on drug development
programs. In September 2005, the Company announced that a preliminary
report of its topical compound for the treatment of diabetic neuropathy was
recently featured in the Journal of Diabetes and Its
Complication. Authored by Dr. C. LeFante and Dr. P. Valensi,
the article appeared in the June 1, 2005 issue, and included findings that
showed the compound reduced the severity of numbness, and irritation from
baseline values. In October 2005, the Company announced the results
of pre-clinical toxicology studies that showed no irritation, photo
toxicity, contact hypersensitivity or photo allergy when applied topically to
hairless guinea pigs and another study that showed no difference in the dermal
response of the compound or placebo when applied to Gottingen Minipigs. (Both
animal models are suggested for the evaluation of topical drugs, by
the FDA). In March 2006, the Company announced the filing of an IND
application with the FDA for its topical compound for
the treatment of Diabetic Peripheral Neuropathy. This
filing allowed the Company to begin human clinical trials following a 30-day
review period. If no further comments were forthcoming from the FDA,
studies with human subjects could commence pending the availability of study
drug. This application included a compilation of all of the
supporting development data and regulatory documentation required to file an IND
application with the FDA. In April 2006, upon FDA approval for its IND, the
Company announced its intent to commence human studies on its
formulation.
The
Company also announced that in anticipation of receiving this IND, it had
previously held its investigators meeting to organize its multi-center Phase II
(b) trials. This would allow the Company to begin these trials as soon as study
drug is available.
In May
2006, the Company announced that it had begun screening patients to start
testing their investigational new drug QR-333 and patients suffering from
diabetic peripheral neuropathy would be given doses in an escalating fashion to
provide pharmacokinetics data.
In
September 2006, the Company announced that the results from its human study,
titled "Single Center, Dose Escalating, Safety, Tolerability, And
Pharmacokinetics Study Of QR-333 In Subjects With Diabetic Peripheral
Neuropathy", demonstrated that QR-333 can be administered safely to patients
suffering from diabetic peripheral neuropathy and it would proceed to conducting
Phase II (b) clinical trials. The essential CMC (Chemistry
Manufacturing and Controls) stage would provide the Company with the necessary
information needed to produce larger quantities of drug for the Phase II (b)
trial involving approximately 180 patients.
The
pharmacokinetics trial was the first study in the U.S. conducted under the FDA
issued IND. The positive data showed that QR-333 is safe, it is not systemically
absorbed and it is well tolerated after multiple doses. These findings are
consistent with prior animal toxicity data and the human Proof of Concept study
performed in France.
In
November 2006, the Company announced that patient enrollment in a Phase II (b)
multi center clinical study of QR-333 for the treatment of symptomatic Diabetic
Peripheral Neuropathy (DPN) had commenced. The Phase II (b) trial
will evaluate the safety and efficacy of QR-333 applied three times daily
compared to placebo-treated patients over 12 weeks. Efficacy will be determined
by Symptom Assessment Scores, a Visual Analogy Scale (VAS), Quality of Life and
Sleep Questionnaires.
Safety will be determined by medical history, physical examination, vital signs,
12-lead ECG, laboratory tests and nerve conduction studies. The study will
involve approximately 140 randomized male and female patients with Type 1 &
2 diabetes, as defined by the ADA (American Diabetes Association) and distal
symmetric diabetic polyneuropathy.
The Study
Chairman is Dr. Philip Raskin, Professor of Medicine University of Texas
Southwestern Medical Center at Dallas,` Texas. The study protocol was approved
by the FDA as a part of Pharma’s IND submission and has been approved by the
required Investigational Review Boards. The completion of the study is dependent
upon enrollment rates that may affect the overall length of the study and the
communication of its results.
In
September 2007, the Company issued an update on a Phase II (b) Clinical Study of
QR-333 on Diabetic Peripheral Neuropathy. The update on the study
noted that over 100 subjects have been enrolled, 52 subjects have completed
treatment and over 225 subjects have been screened for the Phase II (b) study
designed to evaluate the safety and efficacy of the topical formulation on
subjects with diabetic peripheral neuropathy. Subject screening and
enrollment will
continue to ensure an approximately 140 evaluable patient study
population. Once enrolled, subject treatment time is 12
weeks. To date the in-progress safety profile for this study has been
consistent with the findings from the favorable safety results of the
previous human proof of concept study conducted in
France. Subsequently, in March 2008, the Company indicated that the
number of subjects increased in the study.
In
November 2008, the Company announced that the last subject in the Phase IIb
study would complete treatment at the end November 2008 and the study is in the
final stage of data collection, evaluation and study conclusions. The
Company, after collecting all the patient information from 21 study centers and
conferring with its panel of experts on the data, will draft and report study
conclusions, as they are available.
QR-448(a) – In May 2008, the
Company announced positive results from a study conducted in chickens to
evaluate the anti-viral activity of its compound QR448(a). The compound was
administered to chicks that had been infected with Infectious Bronchitis Virus
(IBV). The data from the study indicated that QR448(a) is efficacious against an
IBV challenge in two week old specific pathogen free (SPF) chicks, confirming
previous results indicating that treatment with QR448(a) before or after viral
exposure has the potential to lessen or prevent disease.
The
Company initiated its investigations into the effectiveness of this compound
based on feedback from poultry industry leaders who expressed an increasing need
for additional products to combat IBV. With the completion of this latest study
and the current dossier of data, the Company plans to solicit the poultry
industry for additional guidance and potential interest and opportunities for
developing this compound jointly toward commercialization.
In
September 2008, the Company announced successful results from a follow up study
conducted with its veterinary anti-viral compound QR448(a). The Study
was designed to determine the duration of the anti-viral effect of QR448(a)
against IBV in commercial broiler chickens, a consumer meat type
bird. Results demonstrate longer duration of protection from
infectious bronchitis and reduction of clinical signs in
chickens. Additionally, in September 2008, the Company announced that
the anti-viral compound successfully prevents transmission of infectious
bronchitis in chickens. Veterinary poultry products industry experts
and those familiar with prevention and control of IBV recognize that abating
transmission is perhaps one of the most important ways to economically prevent,
control and manage potential losses due to IBV outbreaks.
The
formulation was initially identified as QR441(a) and for its anti-viral activity
against Highly Pathogenic Avian Influenza H5N1.
QR-336 – In April 2004, the
Company announced the results of a preliminary, pre-clinical animal study which
measured the effect of its proprietary patent applied for formulation against
ionizing (nuclear) radiation. This study determined that parenteral
(injection) administration of the study compound was protective against the
effects of a lethal, whole body ionizing radiation dose in a mouse
model. This compound is being investigated to potentially reduce the
effects of radiation exposure on humans.
In April
2006, the Company announced that it signed an agreement with Dr. William H.
McBride, the Vice Chair of Research, Department of Oncology at UCLA to help
develop an appropriate animal model radio protective research program for QR-336
to comply with New Food and Drug Administration animal efficacy rules for
radio-protective pharmacological compounds.
In
October 2006, the Company announced that it had received significant data
identifying 50 microliters as the least toxic and most effective
radiation protection dose in mice when administered ip (intraperitoneal), po (by
mouth) or sc (under the skin)
prior to radiation exposure. These experiments were essential for providing the
Company with data to optimize the formulation for efficacy and route of
administration, which is required for filing under the FDA’s "Animal Efficacy
Rule".
QR-337 – In September 2003,
the Company announced its intention to file for permission to study its patent
pending potential treatment for psoriasis and other skin
disorders. Continued testing will therefore have to be conducted
under an IND application following positive preliminary results.
QR-435 – In May 2004, the
Company announced that an intranasal spray application of the anti-viral test
compound demonstrated efficacy by significantly reducing the severity of illness
in ferrets that had been infected with the Influenza A virus. In pre-clinical
studies, the antiviral formulation demonstrates antiviral activity against
Ocular and Genital Herpes, indicating a new research and development path for
the versatile compound. The Company is pleased with the progress and indicated
that continued research is required to confirm the compound's safety and
efficacy profiles.
In May
2006, the Company announced that it would begin a series of studies to evaluate
the ocular antiviral efficacy and toxicity of its naturally-derived topical
compound QR-435. Studies will be completed at The Campbell Ophthalmic
Microbiology Laboratory at the University of Pittsburgh in the same lab where
previous successful in vitro studies of QR-435 were performed.
In
December 2006, the Company announced that a series of studies were conducted on
the advice of Campbell Laboratories, University of Pittsburgh, to assess QR-435
(Pharma’s broad spectrum anti-viral) potential for treating Herpes
Keratitis.
While the
in-vitro studies were very successful at killing the herpes virus on direct
contact, the HSV-1/NZW rabbit keratitis model study showed that the compound, in
its aqueous form, did not remain in the eye long enough to penetrate the corneal
epithelial cells where the virus resides in an infection. The HSV-1/NZW rabbit
keratitis model is a recognized standard for evaluating potential therapeutic
agents in this class and is only utilized based on prior positive
experimentation, as was the case.
Pharma
may continue to pursue research and development objectives of this compound in
the treatment of respiratory viruses on the strength of prior successful
in-vitro and ferret model in-vivo studies. The Company's naturally derived
formula has shown significant antiviral properties against various strains of
H3N2 and H5N1 Influenza viruses in these studies.
QR-437 – In January 2004, the
Company reported that its compound, which was demonstrating antiviral activity,
had shown virucidal and virustatic activity against the strain 3B of the Human
Immunodeficiency Virus Type 1 (HIV-1) in an in-vitro
study. Additionally, the Company decided that the derivative
compound of the anti-viral formulation previously found to be effective for
treating Sialorrhea would probably postpone further development on the
Sialorrhea indication and concentrate on further qualification and development
of the anti-viral capabilities of the compound in humans.
QR-439 – In December 2003, the
Company announced positive test results of a preliminary independent in vitro
study indicating that a test compound of the Company previously tested on the
Influenza virus showed “significant virucidal activity against a strain of the
Severe Acute Respiratory Syndrome (SARS) virus.”
In
January 2004, the Company announced that it would conduct two further studies
evaluating the compound which had shown activity against Influenza and
SARS. The first study was intended to repeat the previously announced
results, which demonstrated the compound to be 100 percent effective in
preventing non-infected ferrets in close proximity to an infected ferret from
becoming infected with the Influenza A virus. The second study was a
dose ranging study on the test compound. Upon dosage determination and
confirmation results from these forthcoming animal model studies, a human proof
of concept study using a virus challenge with Influenza A virus in a quarantine
unit would be a viable next step.
QR-440 (a) – The Company received an additional
Investigational New Animal Drug (INAD) number from the Center for Veterinary
Medicine of the FDA. In previous studies, QR-440 has been shown to reduce
inflammation and also suggests possible disease-modifying
potential.
QR-441(a) – In November 2005,
the Company was assigned nine INADs for a broad anti-viral agent by the Center
for Veterinary Medicine of the FDA. Eight of the INADs are for investigating the
compound use against avian flu H5N1 virus in chickens, turkeys, ducks, pigs,
horses, dogs, cats and non-food birds. In January 2006, a ninth INAD
was assigned for investigating its compound for treating arthritis in dogs. In
March 2006, the Company announced that it is planning a series of controlled
experiments designed to test its all natural broad spectrum anti-viral compound
in poultry stocks. The Company also announced that Dr. Timothy S.
Cummings, MS, DVM, ACPV Clinical Poultry Professor at the College of Veterinary
Medicine at Mississippi State University and Thomas G. Voss, Ph.D. Assistant
Professor Tulane University School of Medicine will be assisting the Company in
the development of the INAD bird challenge studies.
In July
2006, the Company announced that it has obtained positive results that support
Pharma's continued progress in developing the natural broad spectrum anti-viral
QR441(a) for use in preventing the spread of avian flu in poultry stocks. The
results of the healthy chicken medical feed study confirmed that food or water
dose forms provide an opportunity for potential commercialization if the
compound demonstrates efficacy within these dose forms. The results
clearly showed that the chickens tolerated and consumed all concentrations of
QR441 (a) in the medicated feed. They also tolerated and consumed the low
concentration of drug in the medicated water.
In
January 2007, the Company announced positive results from a study evaluating its
anti-viral compound QR-441(a) in embryonating egg and VERO E6 cell test models.
The preliminary study demonstrated QR-441(a) as a potential antiviral agent in
reducing Infectious Bronchitis and New Castle Disease, two viral poultry
diseases that have a significant economic impact to the poultry industry on an
annual basis. Previous in vitro studies have demonstrated that QR-441(a) to be a
potent antiviral agent against H5N1 (Avian Flu).
In
February 2007, the Company announced that it had signed an agreement with the
State of Israel Ministry of Agriculture & Rural Development (MOAG) and the
Kimron Veterinary Institute to conduct a clinical trial testing the anti-viral
capacity of the Company’s compound QR-441(a) administered as a medical feed and
water to chickens exposed to HPAI (Highly Pathogenic Avian Influenza)
H5N1.
If
successful this study could potentially provide data on the efficacy of
QR-441(a) in preventing the infection of food grade poultry through the use of
formulated feed and water. Positive data could be used to continue the
development of the compound in the U.S with guidance from the FDA under the
INAD’s issued to the Company in 2005 and might also be useful for development
outside the United States, where the impact of disease has already been
felt. See also QR-448(a).
QR-443 – In August 2006, the
Company announced that it had obtained positive results for its QR-443 compound
for the treatment of Cachexia. Cachexia is an extremely debilitating
and life threatening, wasting syndrome associated with chronic diseases such as
cancer, AIDS, chronic renal failure, COPD and rheumatoid arthritis, where
inflammation has a significant impact and patients’ experience loss of weight,
muscle atrophy, fatigue, weakness and decreased appetite. The results of an
animal study found a 75% efficacy rate in the treatment of mice with this
condition.
In
January 2007, the Company announced that it had completed a preliminary follow
up Cachexia study, evaluating weight loss in mice. The tumor burden Cachexia
model study concluded that QR-443 was as effective in delaying the progression
of Cachexia when given orally as it had been shown to be when administered
intra-peritoneally in a previous study.
The new
data compliments the previous study results demonstrating a correlation between
effectiveness and the frequency of administration of the QR-443
compound.
On June
20, 2007, the Company announced that it had completed a follow-up study to
evaluate the impact of QR-443 on levels of a pro-inflammatory cytokine
Interleukin-6 (IL-6) in a cachexia model. This new data concluded that
responding mice had lower levels of serum IL-6 when administered QR-443 orally
than mice that received placebo. This reduction in IL-6 suggests a method of
action for the delayed onset and reduced severity of cachexia observed in this
study as well as the previously conducted cachexia model study.
QR-449 – In July 2007, the
Company announced that it had initiated a human clinical safety trial to
evaluate the effects of QR-449 on subjects with Metabolic
Syndrome. The primary objectives for the studies are to determine the
safety of QR-449 when administered in a range dosing fashion and determine the
effects of QR-449 on metabolic imbalances.
QR-340 – On February 24, 2009,
the Quigley Corporation announced that it had signed a license with assignment
of ownership agreement for its patented formulation QR-340 developed by its
wholly owned subsidiary, Quigley Pharma Inc. The compound has been clinically
tested and shown to improve the appearance of scars in a comparative study. The
Agreement is with Levlad, LLC/Natures Gate, a manufacturer and marketer of
personal care products based on botanicals. The general terms of the
agreement allow the assignee to further refine, develop and commercialize the
product with exclusivity and eventual full ownership of the patent within five
years, beginning January 2009. The agreement is based on required royalty
payments totaling $1.1 million to The Quigley Corporation over the time period.
Under the terms of the agreement, if the minimum payments and terms are not met
within the five-year period, The Company retains full rights and ownership of
the property. However, Levlad can continue to pay per unit royalties beyond five
years for a non-exclusive license.
Health And
Wellness
On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc., whose
major shareholder is Mr. Kevin P. Brogan, the then president of
Darius. Darius marketed health and wellness products through its
wholly-owned subsidiary, Innerlight Inc. that constituted the Health and
Wellness segment of the Company. The terms of the sale agreement
included a cash purchase price of $1,000,000 by InnerLight Holdings, Inc. for
the stock of Darius and its subsidiaries without guarantees, warranties or
indemnifications. Financial information related to this former
segment is presented as Discontinued Operations. See discussion in
Note 3 to Consolidated Financial Statements.
Patents, Trademarks, Royalty
and Commission Agreements
The
Company currently owns no patents for cold-remedy products. However,
the Company has been assigned patent applications which are hereinafter
discussed and has been granted an exclusive agreement for worldwide
representation, manufacturing, marketing and distribution rights to a zinc
gluconate glycine lozenge formulation, which are patented as
follows:
|
United
States:
|
No.
4 684 528 (August 4, 1987, expired August 2004)
|
|
No.
4 758 439 (July 19, 1988, expired August 2004)
|
|
|
Canada:
|
No.
1 243 952 (November 1, 1988, expired June 2005)
|
|
Great
Britain:
|
No.
2 179 536 (December 21, 1988, expired June 2005)
|
|
Germany:
|
No.
3,587,766 (March 2, 1994, expired June 2005)
|
|
Sweden:
|
No. 0 183 840 (March 2, 1994,
expired June 2005)
|
|
France
& Italy:
|
No.
EP 0 183 840 B1 (March 2, 1994, expired June
2005)
Japan:
Pending
|
The
following patents have been assigned to the Company in relation to Pharma, together with issue
date:
|
United
States:
|
No.
6 555 573 B2 (April 29, 2003)
|
No.
6 592 896 B2 (July 15, 2003)
|
||
|
No.
6 596 313 B2 (July 22, 2003)
|
No.
6 753 325 B2 (June 22, 2004)
|
|||
|
No.
6 827 945 B2 (December 7, 2004)
|
No.
7,083,813 B2 (August 1, 2006)
|
|||
|
No.
7,166,435 B2 (January 23, 2007)
|
No.
7,175,987 B2 (February 13, 2007)
|
|||
|
No.
7,396,546 B2 (July 8, 2008)
|
No.
7,399,783 B2 (July 15, 2008)
|
|||
|
No.
7,405,046 B2 (July 29, 2008)
|
No.
7,410,659 B2 (August 12, 2008)
|
|||
|
No.
7,435,725 B2 (October 14, 2008)
|
||||
|
Mexico
|
No.
236311 (April 28, 2006)
|
South
Africa
|
No.
2003/4247 (July 28, 2004)
|
|
|
Mexico
|
No.
259329 (August 4, 2006)
|
South
Africa
|
No.
2003/9802 (July 28, 2004)
|
|
|
South
Africa
|
No.
2004/4614 (October 28, 2005)
|
|||
|
New
Zealand
|
No.
533439 (October 12, 2006)
|
South
Africa
|
No.
2005/0517 (December 28, 2005)
|
|
|
New
Zealand
|
No.
526041 (May 12, 2005)
|
South
Africa
|
No.
2004/3365 (May 31, 2006)
|
|
|
New
Zealand
|
No.
530187 (June 7, 2007)
|
South
Africa
|
No.
2004/3364 (October 25, 2006)
|
|
|
New
Zealand
|
No.
537821 (September 13, 2007)
|
|||
|
New
Zealand
|
No.
532775 (February 8, 2007)
|
Israel
|
No.
159357 (November 21, 2006)
|
|
|
Australia
|
No.
2002231095 (November 24, 2005)
|
Australia
|
No.
2002232464 (February 22, 2007)
|
|
|
Australia
|
No.
2002352501 (July 5, 2007)
|
Australia
|
No.
2002365155 (January 31, 2008)
|
|
|
Australia
|
No.
2002309615 (January 31, 2008)
|
|||
|
India
|
No.
00004/MUMP/2004 (December 27, 2007)
|
|||
The
Cold-EezeÒ products
are marketed by the Company in accordance with the terms of a licensing
agreement (between the Company and the developer). The contract is
assignable by the Company with the developer’s consent. In return for
exclusive distribution rights, the Company paid the developer a 3% royalty and a
2% consulting fee based on sales collected, less certain deductions, throughout
the term of this agreement, which expired in 2007. However, the
Company and the developer are in litigation and as such no potential offset for
these fees from such litigation has been recorded.
During
1997, the Company obtained a trademark for the major components of its lozenge,
ZIGGÔ (denoting zinc
gluconate glycine), to set Cold-Eeze® apart from the imitations then
proliferating the marketplace.
An
agreement between the Company and its founders was entered into on June 1,
1995. The founders, both officers and stockholders of the Company, in
consideration of the acquisition of the Cold-EezeÒ cold therapy product, have
received a total commission of five percent (5%), on sales collected, less
certain deductions. This agreement expired on May 31,
2005.
Product Distribution and
Customers
The
Company has several Broker, Distributor and Representative Agreements, both
nationally and internationally, which provide for commission compensation based
on sales performance.
The
Cold-EezeÒ products
are distributed through numerous food, chain drug and mass merchandisers
throughout the United States, including: Walgreen Co., Wal-Mart, Ahold, Super
Valu, CVS, RiteAid, Publix, Winn-Dixie Stores, Inc., Target, The Kroger Company,
Safeway Inc., Kmart Corporation, and wholesale distributors including,
AmerisourceBergen and Cardinal Distribution.
The
Company is not dependent on any single customer as the broad range of customers
includes many large wholesalers, ass
merchandisers, and multi-outlet pharmacy chains, five of which account for a
significant percentage of sales volume. The top five customers of the
Company represent 48%, 49%, and 47% of its continuing consolidated gross
revenues for the years ended December 31, 2008, 2007 and 2006,
respectively.
Pharma
currently has no sales since it is undergoing research and development activity
in compliance with regulatory requirements and is at the initial stages of what
may be a lengthy process to develop commercial products.
On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc., whose
major shareholder is Mr. Kevin P. Brogan, the then president of
Darius. Darius marketed health and wellness products through its
wholly-owned subsidiary, Innerlight Inc. that constituted the Health and
Wellness segment of the Company. The terms of the sale agreement
included a cash purchase price of $1,000,000 by InnerLight Holdings, Inc. for
the stock of Darius and its subsidiaries without guarantees, warranties or
indemnifications. Financial information related to this former
segment is presented as Discontinued Operations. See discussion in
Note 3 to Consolidated Financial Statements.
Research and
Development
The
Company’s research and development costs for the years ended December 31, 2008,
2007 and 2006 were $4,241,724, $6,482,485 and $3,787,498
respectively. Future research and development expenditures are
anticipated in order to develop extensions of the Cold-EezeÒ product, including
potential unrelated new products in the consumer health care industry, that are
primarily supported by clinical studies, for efficacious long-term products that
can be coupled with possible line extension derivatives for a family of
products. Clinical studies and testing are anticipated in connection
with Pharma, such as the formulation of products for diabetic use, radiation
dermatitis, influenza A, arthritis and other disorders. Pharma is
currently involved in research activity following patent applications that have
been assigned to the Company. Research and development costs,
relating to potential products, may increase significantly over time as
milestones in the development and regulatory process may be
achieved.
Regulatory
Matters
The
business of the Company is subject to federal and state laws and regulations
adopted for the health and safety of users of the Company’s
products. The Company’s Cold-EezeÒ product is a homeopathic
remedy, which is subject to regulation by various federal, state and local
agencies, including the FDA and the Homeopathic Pharmacopoeia of the United
States. These regulatory authorities have broad powers, and the
Company is subject to regulatory and legislative changes that can affect the
economics of the industry by requiring changes in operating practices or by
influencing the demand for, and the costs of, providing its
products. Management believes that the Company is in compliance with
all such laws, regulations and standards currently in effect including the Food,
Drug and Cosmetics Act of 1938 and the Homeopathic Pharmacopoeia Regulatory
Service. Although it is possible that future results of operations
could be materially affected by the future costs of compliance, management
believes that the future costs will not have a material adverse effect on the
Company’s financial position or competitive position.
The
pre-clinical development, clinical trials, product manufacturing and marketing
of Pharma's
potential new products are subject to federal and state regulation in the United
States and other countries. Obtaining FDA regulatory approval for these
pharmaceutical products can require substantial resources and take several
years. The length of this process depends on the type, complexity and
novelty of the product and the nature of the disease or other indications to be
treated. If the Company cannot obtain regulatory approval of these new products
in a timely manner or if the patents are not granted or if the patents are
subsequently challenged, these possible events could all have a material effect
on the business and financial condition of the Company. The strength
of the Company’s patent position may be important to its long-term
success. There can be no assurance that these patents and patent
applications will effectively protect the Company’s products from duplication by
others.
Competition
The
Company competes with other suppliers of cold-remedy products. These
suppliers range widely in size. Some of the Company’s competitors
have significantly greater financial, technical or marketing resources than the
Company. Management believes that its Cold-Eeze® product, which has
been clinically proven in two double-blind studies to reduce the severity and
duration of common cold symptoms, offers a significant advantage over many of
its competitors in the over-the-counter cold-remedy market. The
Company believes that its ability to compete depends on a number of factors,
including price, product quality, availability, speed to market, reliability,
credit terms, name recognition, delivery time and post-sale service and
support. Effective October 1, 2004, a subsidiary of the Company
commenced manufacturing the Cold-EezeÒ lozenge
product. This subsidiary assures future production capabilities of
the lozenge product which constitutes primarily all of the cold remedy
revenue.
Employees
At
December 31, 2008 the Company employed 86 full-time persons, the majority of
which were employed at the Company’s manufacturing facility in a production
function. The remainder were involved in an executive, marketing or
administrative capacity. None of the Company’s employees are covered
by a collective bargaining agreement or are members of a union.
Suppliers
Prior to
October 1, 2004, the manufacturing of the lozenge form of Cold-EezeÒ was outsourced, but is now
under the control of the Company. The other forms of Cold-EezeÒ and remaining products of
the cold remedy segment continue to be manufactured by contract
manufacturers. Should these third party relationships terminate or
discontinue for any reason, the Company has formulated a contingency plan
necessary in order to prevent such discontinuance from materially affecting the
Company’s operations. Any such termination may, however, result in a temporary
delay in production until the replacement facility is able to meet the Company’s
production requirements.
Raw
materials used in the production of the cold-remedy products are available from
numerous sources. Currently, they are being procured from a single
vendor in order to secure purchasing economies and qualitative
security. In a situation where this one vendor is not able to supply
the ingredients, other sources have been identified. Any situation
where the vendor is not able to supply the contract manufacturer with
ingredients may result in a temporary delay in production until replacement
supplies are obtained to meet the Company’s production
requirements.
Website
Access
The
Company’s website address is www.quigleyco.com. The
Company’s filings with the SEC are available at no cost on its website as soon
as practicable after filing of such reports with the SEC.
ITEM
1A. RISK
FACTORS
The
Company Has A History of Losses and Limited Working Capital and Expects to
Increase Spending.
The
Company has experienced net losses for four of the past seven fiscal
years. Although the Company earned net income of approximately
$3,217,000, $453,000 and $675,000, respectively, in the fiscal years ended
December 31, 2005, December 31, 2004 and 2003, it incurred net losses of
$5,534,000, $2,458,000, $1,748,000, and $6,454,000, respectively, in
the fiscal years ended December 31, 2008, December 31, 2007, December 31, 2006,
December 31, 2002. As of December 31, 2008, The Company had working
capital of approximately $14,072,000. Since the Company continues to
spend significant amounts on research and development in connection with
Pharma’s product development, it is uncertain whether the Company will generate
sufficient revenues to meet expenses or to operate profitably in the
future.
The Company Holds Patents Which It
May Not Be Able to Develop Into Pharmaceutical Medications.
Future
success depends in part on Pharma’s ability to research and develop prescription
medications based on patents, which currently are:
|
·
|
A
Patent (No. 6,555,573 B2) entitled “Method and Composition for the Topical
Treatment of Diabetic Neuropathy.” The patent extends through March 27,
2021.
|
|
·
|
A
Patent (No. 6,592,896 B2) entitled “Medicinal Composition and Method of
Using It” (for Treatment of Sialorrhea and other Disorders) for a product
to relieve sialorrhea (drooling) in patients suffering from Amyotrophic
Lateral Sclerosis (ALS), otherwise known as Lou Gehrig’s
Disease. The patent extends through August 5,
2021.
|
|
·
|
A
Patent (No. 6,596,313 B2) entitled “Nutritional Supplement and Method of
Using It” for a product to relieve sialorrhea (drooling) in patients
suffering from Amyotrophic Lateral Sclerosis (ALS), otherwise known as Lou
Gehrig’s Disease. The patent extends through April 14,
2022.
|
|
·
|
A
Patent (No. 6,753,325 B2) entitled “Composition and Method for Prevention,
Reduction and Treatment of Radiation Dermatitis,” a composition for
preventing, reducing or treating radiation dermatitis. The
patent extends through November 5,
2021.
|
|
·
|
A
Patent (No. 6,827,945 B2) entitled “Nutritional Supplements and Method of
Using Same” for a method for treating at least one symptom of
arthritis. The patent extends through April 22,
2023.
|
|
·
|
A
Patent (No. 7,083,813 B2) entitled “Methods for The Treatment of
Peripheral Neural and Vascular Ailments.” The patent extends
through August 4, 2023.
|
|
·
|
A
Patent (No. 7,166,435 B2) entitled “Compositions and Methods for Reducing
the Tranmissivity of Illnesses.” This patent will provide
additional protection to an existing composition patent (number
6,592,896), which the Company received in July 2003 and will support
on-going investigations and potential commercialization opportunities. The
Company will be continuing its studies to test the effects of the
referenced compound against avian flu and human influenza. The patent
extends through November 5, 2021.
|
|
·
|
A
Patent (No. 7,175,987 B2) entitled “Compositions and Methods for The
Treatment of Herpes.” The patent extends through November 5,
2021.
|
|
·
|
A
Patent (No. 7,396,546 B2) entitled “Anti-Microbial Compositions and
Methods of Using Same” The patent extends through August 6,
2021.
|
|
·
|
A
Patent (No. 7,399,783 B2) entitled “Methods for the Treatment of Scar
Tissue.” The patent extends through September 4,
2026.
|
|
·
|
A
Patent (No. 7,405,046 B2) entitled “Compositions and Methods for Treatment
of Rhinovirus.” The patent extends through August 6,
2021.
|
|
·
|
A
Patent (No. 7,410,659 B2) entitled “Methods for the Treatment of
Peripheral Neural and Vascular Ailments.” The patent extends
through November 6, 2022.
|
|
·
|
A
Patent (No. 7,435,725 B2) entitled “Oral Compositions and Methods for
Prevention, Reduction and Treatment of Radiation Injury.” The
patent extends through January 14,
2022.
|
|
·
|
A
Mexican Patent (No. 236311) entitled “Method and Composition for the
Treatment of Diabetic Neuropathy.” The patent extends through
December 18, 2020.
|
|
·
|
A
Mexican Patent (No. 259329) entitled “Nutritional Supplements and Methods
for Prevention, Reduction and Treatment of Radiation Injury” the patent
extends through April 30, 2022.
|
|
·
|
A
New Zealand Patent (No. 533439) entitled “Methods for The Treatment of
Peripheral Neural and Vascular Ailments.” The patent extends
through November 6, 2022.
|
|
·
|
A
New Zealand Patent (No. 526041) entitled “Method and Composition for the
Treatment of Diabetic Neuropathy.” The patent extends through December 18,
2021.
|
|
·
|
A
New Zealand Patent (No. 530187) entitled “Nutritional Supplements and
Methods of Using Same.” The patent extends through August 6,
2022.
|
|
·
|
A
New Zealand Patent (No. 537821) entitled “Anti-Microbial Compositions and
Methods of Using Same.” The patent extends through July 23,
2023.
|
|
·
|
A
New Zealand Patent (No. 532775) entitled “Topical Compositions and Methods
for Treatment of Adverse Effects of Ionizing Radiation,” The patent
extends through November 6, 2022.
|
|
·
|
An
Australian Patent (No. 2002231095) entitled “Method and Composition for
the Treatment of Diabetic Neuropathy.” The patent
extends through December 18, 2021.
|
|
·
|
An
Australian Patent (No. 2002352501) entitled “Method for The Treatment of
Peripheral Neural and Vascular Ailments.” The
patent extends through November 5,
2022.
|
|
·
|
An
Australian Patent (No. 2002232464) entitled “Nutritional Supplements and
Methods of Using Same.” The patent extends through
August 5, 2022.
|
|
·
|
An
Australian Patent (No. 2002365155) “Topical Compositions and Methods for
Treatment of Adverse Effects of Ionizing Radiation,” the patent extends
through November 5, 2022.
|
|
·
|
An
Australian Patent (No. 2002309615) “Nutritional Supplements and Methods
for Prevention, Reduction and Treatment of Radiation Injury” the patent
extends through April 30, 2022.
|
|
·
|
A
South African Patent (No. 2003/4247) entitled “Methods and Composition for
the Treatment of Diabetic Neuropathy.” The patent
extends through December 18, 2021.
|
|
·
|
A
South African Patent (No. 2004/3364) “Nutritional Supplements and Methods
for Prevention, Reduction and Treatment of Radiation Injury” the patent
extends through May 1, 2022.
|
|
·
|
A
South African Patent (No. 2003/9802) entitled “Nutritional Supplements and
Methods of Using Same” for a method for treating at least one symptom of
arthritis. The patent extends through August 5,
2022.
|
|
·
|
A
South African Patent (No. 2004/4614) entitled “Methods for The Treatment
of Peripheral Neural and Vascular Ailments.” The patent extends
through November 5, 2022.
|
|
·
|
A
South African Patent (No. 2005/0517) entitled “Anti-Microbial Compositions
& Methods for Using Same,” the patent extends through July 23,
2023.
|
|
·
|
A
South African Patent (No. 2004/3365) “Topical Compositions and Methods for
Treatment of Adverse Effects of Ionizing Radiation,” the patent extends
through November 5, 2022.
|
|
·
|
An
Israeli Patent (No. 159357) entitled “Nutritional Supplements and Methods
of Using Same,” the patent extends through August 6,
2022.
|
|
·
|
An
Indian Patent (No. 00004/MUMP/2004) entitled “A Nutritional
Supplement.” The patent extends through August 6,
2022.
|
These
potential new products are in the development stage and no assurances can be
given that commercially viable products will be developed from these patent
applications. Prior to any new product being ready for sale,
substantial resources will have to be committed for research, development,
preclinical testing, clinical trials, manufacturing scale-up and regulatory
approval. The Company faces significant technological risks inherent
in developing these products. The Company may abandon some or all of
the proposed new products before they become commercially
viable. Even if the Company develops and obtains approval of a new
product, if the Company cannot successfully commercialize it in a timely manner,
its business and financial condition may be materially adversely
affected.
The
Company Will Need To Obtain Additional Capital To Support Long-Term Product
Development And Commercialization Programs.
The
Company’s ability to achieve and sustain operating profitability depends in
large part on the ability to commence, execute and complete clinical programs
for, and obtain additional regulatory approvals for, prescription medications
developed by Pharma, particularly in the U.S. and Europe. There is no
assurance that the Company will ever obtain such approvals or achieve
significant levels of sales. The current sales levels of Cold-Eeze®
products may not generate all the funds the Company anticipates will be needed
to support current plans for product development.
The
Company may need to obtain additional financing to support its long-term product
development and commercialization programs. Additional funds may be
sought through public and private stock offerings, arrangements with corporate
partners, borrowings under lines of credit or other sources. Access
to, and availability of, funding for such activities may prove difficult or
unattainable due to several factors including weak current and future economic
conditions, reduction in the availability of credit, financial market volatility
and recession.
The
amount of capital that may be needed to complete product development of Pharma’s
potential products will depend on many factors, including;
|
|
·
|
the
cost involved in applying for and obtaining FDA and international
regulatory approvals;
|
|
|
·
|
whether
the Company elects to establish partnering arrangements for development,
sales, manufacturing and marketing of such
products;
|
|
|
·
|
the
level of future sales of Cold-Eeze® products, and expense levels for
international sales and marketing
efforts;
|
|
|
·
|
whether
the Company can establish and maintain strategic arrangements for
development, sales, manufacturing and marketing of its products;
and
|
|
|
·
|
whether
any or all of the outstanding options are exercised and the timing and
amount of these exercises.
|
Many of
the foregoing factors are not within the Company’s control. If
additional funds are required and such funds are not available on reasonable
terms, the Company may have to reduce its capital expenditures, scale back its
development of new products, reduce its workforce and out-license to others,
products or technologies that the Company otherwise would seek to commercialize
itself. Any additional equity financing will be dilutive to
stockholders, and any debt financing, if available, may include restrictive
covenants.
The Company’s Products and Potential
New Products Are Subject to Extensive Governmental Regulation.
The
Company’s business is regulated by various agencies of the states and localities
where its products are sold. Governmental regulations in foreign
countries where the Company plans to commence or expand sales may prevent or
delay entry into a market or prevent or delay the introduction, or require the
reformulation, of certain of its products. In addition, no prediction
can be made as to whether new domestic or foreign legislation regulating our
activities will be enacted. Any new legislation could have a material
adverse effect on its business, financial condition and
operations. Non-compliance with any applicable requirements may
subject the Company or the manufacturers of its products to sanctions, including
warning letters, fines, product recalls and seizures.
Cold Remedy
Products. The manufacturing, processing, formulation,
packaging, labeling and advertising of the cold remedy products are subject to
regulation by several federal agencies, including:
· the
FDA;
· the
Federal Trade Commission (“FTC”);
· the
Consumer Product Safety Commission;
· the
United States Department of Agriculture;
· the
United States Postal Service;
· the
United States Environmental Protection Agency; and
· the
Occupational Safety and Health Administration.
In
particular, the FDA regulates the safety, labeling and distribution of dietary
supplements, including vitamins, minerals and herbs, food additives, food
supplements, over-the-counter and prescription drugs and
cosmetics. The FTC also has overlapping jurisdiction with the FDA to
regulate the promotion and advertising of vitamins, over-the-counter drugs,
cosmetics and foods. In addition, the cold remedy products are
homeopathic remedies which are regulated by the Homeopathic Pharmacopoeia of the
United States (“HPUS”). HPUS sets the standards for source,
composition and preparation of homeopathic remedies which are officially
recognized in the Federal Food, Drug and Cosmetics Act of 1938.
Pharma. The
preclinical development, clinical trials, product manufacturing and marketing of
Pharma’s potential new products are subject to federal and state regulation in
the United States and other countries. Clinical trials and product
marketing and manufacturing are subject to the rigorous review and approval
processes of the FDA and foreign regulatory authorities. Obtaining
FDA and other required regulatory approvals is lengthy and
expensive. Typically, obtaining regulatory approval for
pharmaceutical products requires substantial resources and takes several
years. The length of this process depends on the type, complexity and
novelty of the product and the nature of the disease or other indication to be
treated. Preclinical studies must comply with FDA
regulations. Clinical trials must also comply with FDA regulations
and may require large numbers of test subjects, complex protocols and possibly
lengthy follow-up periods. Consequently, satisfaction of government
regulations may take several years, may cause delays in introducing potential
new products for considerable periods of time and may require imposing costly
procedures upon the Company’s activities. If regulatory approval of
new products is not obtained in a timely manner or not at all, the Company could
be materially
adversely affected. Even if regulatory approval of new products is
obtained, such approval may impose limitations on the indicated uses for which
the products may be marketed which could also materially adversely affect the
business, financial condition and future operations of the Company.
The Company’s Business is Very
Competitive and Increased Competition Could Have a Significant Impact on
Earnings.
Both the
non-prescription healthcare product and pharmaceutical industries are highly
competitive. Many of the Company’s competitors have substantially
greater capital resources, research and development staffs, facilities and
experience than it does. These and other entities may have or may
develop new technologies. These technologies may be used to develop
products that compete with the Company.
The
Company believes that the primary cold remedy product, Cold-Eeze®, has a
competitive advantage over other cold remedy products because it has been
clinically proven to reduce the severity and duration of common cold
symptoms. Competition in Pharma’s potential product areas would most
likely come from large pharmaceutical companies as well as other companies,
universities and research institutions, many of which have resources far in
excess of the Company’s resources.
The
Company believes that its ability to compete depends on a number of factors,
including price, product quality, availability, reliability and name recognition
of its cold remedy products and Pharma’s ability to successfully develop and
market prescription medications. There can be no assurance that the
Company will be able to compete successfully in the future. If the
Company is unable to compete, its earnings may be significantly
impacted.
The Company’s Future Success is
Dependent on the Continued Services of Key Personnel Including The Chairman of
the Board of Directors, President and Chief Executive
Officer.
The
Company’s future success depends in large part on the continued service of key
personnel. In particular, the loss of the services of Guy J. Quigley,
Chairman of the Board, President and Chief Executive Officer could have a
material adverse effect on operations. The Company had an employment
agreement with Mr. Quigley which expired on December 31, 2005. Future
success and growth also depends on the Company’s ability to continue to attract,
motivate and retain highly qualified employees. If the Company is
unable to attract, motivate and retain qualified employees, our business and
operations could be materially adversely affected.
The Company’s Future Success Depends
on the Continued Employment of Richard A. Rosenbloom, M.D., Ph.D., with
Pharma.
Pharma’s
potential new products are being developed through the efforts of Dr.
Rosenbloom. The loss of his services could have a material adverse
effect on the Company’s product development and future operations.
The
Company’s Future Success Depends on the Continued Sales of its Principal
Product.
For the
fiscal year ended December 31, 2008, the Cold-Eeze® products represented
approximately 89% of the Company’s total sales. The Cold-Eeze®
products continues to be a major part of its business. Accordingly,
the Company has to depend on the continued acceptance of Cold-Eeze® products by
its customers. However, there can be no assurance that the Cold-Eeze®
products will continue to receive market acceptance. The inability to
successfully commercialize Cold-Eeze® in the future, for any reason, would have
a material adverse effect on the financial condition, prospects and ability to
continue operations of the Company.
The
Company Has a Concentration of Sales to, and Accounts Receivable from, Several
Large Customers.
Although
the Company has a broad range of customers that includes many large wholesalers,
mass merchandisers and multiple outlet pharmacy chains, the five largest
customers account for a significant percentage of sales. These five
customers accounted for 48% of total sales for the fiscal year ended December
31, 2008 and 49% of total sales for the fiscal year ended December 31,
2007. In addition, customers comprising the five largest accounts
receivable balances represented 55% and 40% of total accounts receivable
balances at December 31, 2008 and 2007, respectively. Credit is
extended to customers based upon an evaluation of their financial condition and
credit history, and collateral is not generally required. If one or
more of these large customers cannot pay, the write-off of their accounts
receivable would have a material adverse effect on the Company’s operations and
financial condition. The loss of sales to any one or more of these
large customers would also have a material adverse effect on the operations and
financial condition of the Company.
The
Company is Dependent on Third-Party Manufacturers and Suppliers for Certain of
the Cold Remedy Products.
All
active ingredients that are raw materials used in connection with the Cold-Eeze®
product are purchased from a single unaffiliated supplier. Should any
of these relationships terminate, the Company believes that the contingency
plans which have been formulated would prevent a termination from materially
affecting its operations. However, if any of these relationships are
terminated, there may be delays in production of the Company’s products until an
acceptable replacement facility is located. The Company continues to
look for safe and reliable multiple-location sources for products and raw
materials so that it can continue to obtain products and raw materials in the
event of a disruption in its business relationship with any single manufacturer
or supplier. While secondary sources have been identified for some of
the Company’s products and raw materials, its inability to find other sources
for some of its other products and raw materials may have a material adverse
effect on its operations. In addition, the terms on which
manufacturers and suppliers will make products and raw materials available to us
could have a material effect on the Company’s success.
The Company is Uncertain as to
Whether It Can Protect Its Proprietary Rights.
The
strength of the Company’s patent position may be important to its long-term
success. The Company currently owns thirteen U.S. and twenty foreign
patents in connection with potential products that are being developed by
Pharma. In addition, the Company has been granted an exclusive
agreement for worldwide representation, manufacturing, marketing and
distribution rights to a zinc/gluconate/glycine lozenge
formulation. That formulation has been patented in the United States,
Germany, France, Italy, Sweden, Canada and Great Britain and a patent is pending
in Japan. However, this patent in the United States expired in August
2004 and expired in June 2005 in all countries except Japan.
There can
be no assurance that these patents and the Company’s exclusive license will
effectively protect its products from duplication by others. In
addition, the Company may not be able to afford the expense of any litigation
which may be necessary to enforce its rights under any of the
patents. Although the Company believes that current and future
products do not and will not infringe upon the patents or violate the
proprietary rights of others, if any of the current or future products do
infringe upon the patents or proprietary rights of others, the Company may have
to modify the products or obtain an additional license for the manufacture
and/or sale of such products. The Company could also be prohibited
from selling the infringing products. If the Company is found to
infringe on the proprietary rights of others, it is uncertain whether the
Company will be able to take corrective actions in a timely manner, upon
acceptable terms and conditions, or at all, and the failure to do so could have
a material adverse effect upon its business, financial condition and
operations.
The
Company also uses non-disclosure agreements with its employees, suppliers,
consultants and customers to establish and protect the ideas, concepts and
documentation of its confidential non-patented and non-copyright protected
proprietary technology and know-how. However, these methods may not
afford complete protection. There can be no assurance that third
parties will not obtain access to or independently develop the Company’s
technologies, know-how, ideas, concepts and documentation, which could have a
material adverse effect on the Company’s financial condition.
The Sales of the Company’s Primary
Product Fluctuates by Season.
A
significant portion of the Company’s business is highly seasonal, which causes
major variations in operating results from quarter to quarter. The
third and fourth quarters generally represent the largest sales volume for the
cold remedy products. There can be no assurance that the Company will
be able to manage its working capital needs and its inventory to meet the
fluctuating demand for these products. Failure to accurately predict
and respond to consumer demand may result in the production of excess
inventory. Conversely, if products achieve greater success than
anticipated for any given quarter, this may result in insufficient inventory to
meet customer demand.
The Company’s Existing Products and
Potential New Products Under Development Expose the Company to Potential Product
Liability Claims.
The
Company’s business results in exposure to an inherent risk of potential product
liability claims, including claims for serious bodily injury or death caused by
the sales of the Company’s existing products and the clinical trials of products
which are being developed. These claims could lead to substantial
damage awards. The Company currently maintains product liability
insurance in the amount of, and with a maximum payout of $25
million. A successful claim brought against the Company in excess of,
or outside of, existing insurance coverage could have a material adverse effect
on the Company’s results of operations and financial
condition. Claims against the Company, regardless of their merit or
eventual outcome, may also have a material adverse effect on the consumer demand
for its products.
The Company is Involved in Lawsuits
Regarding Claims Relating to Certain of the Cold-Eeze® Products and other
Business Matters.
The
Company is, from time to time, subject to various legal proceedings and claims,
either asserted or unasserted. Any such claims, including those
contained in Item 3 of this report, whether with or without merit, could be
time-consuming and expensive to defend and could divert management's attention
and resources. While management believes that the Company has
adequate insurance coverage and, if applicable, accrued loss contingencies for
all known matters, there is no assurance that the outcome of all current or
future litigation will not have a material adverse effect on the
Company.
A Substantial Amount of the Company’s
Outstanding Common Stock is Owned by the Chairman of the Board and President and
Executive Officers and Directors, as a Group Can Significantly Influence All
Matters Voted on by Stockholders.
Guy J.
Quigley, Chairman of the Board, President and Chief Executive Officer, through
his beneficial ownership, has the power to vote approximately 25.4% of The Company’s common
stock. Mr. Quigley and the other executive officers and directors
collectively beneficially own approximately 36.7% of the common
stock. These individuals have significant influence over the outcome
of all matters submitted to stockholders for approval, including election of
directors. Consequently, they exercise substantial control over all
major decisions which could prevent a change of control of the
Company.
The Company’s Stock Price is
Volatile.
The
market price of the Company’s common stock has experienced significant
volatility. From January 1, 2005 to January 31, 2009, the per share
bid price has ranged from a low of approximately $2.85 to a high of
approximately $16.94. There are several factors which could affect
the price of the common stock, some of which are announcements of technological
innovations for new commercial products by us or competitors, developments
concerning propriety rights, new or revised governmental regulation or general
conditions in the market for the Company’s products. Sales of a
substantial number of shares by existing stockholders could also have an adverse
effect on the market price of the common stock.
Future
Sales of Shares of the Company’s Common Stock in the Public Market Could
Adversely Affect the Trading Price of Shares of the Common Stock and the
Company’s Ability to Raise Funds in New Stock Offerings.
Future
sales of substantial amounts of shares of the Company’s common stock in the
public market, or the perception that such sales are likely to occur, could
affect prevailing trading prices of the common stock. As of December
31, 2008, the Company had 12,908,383 shares of common stock
outstanding.
The
Company also has outstanding options to purchase an aggregate of 2,268,250
shares of common stock at an average exercise price of $7.76 per
share. If the holders of these options were to attempt to sell a
substantial amount of their holdings at once, the market price of the common
stock would likely decline. Moreover, the perceived risk of this
potential dilution could cause stockholders to attempt to sell their shares and
investors to “short” the stock, a practice in which an investor sells shares
that he or she does not own at prevailing market prices, hoping to purchase
shares later at a lower price to cover the sale. As each of these
events would cause the number of shares of common stock being offered for sale
to increase, the common stock’s market price would likely further
decline. All of these events could combine to make it very difficult
for the Company to sell equity or equity-related securities in the future at a
time and price that it deems appropriate.
The Company Does Not Intend to Pay
Cash Dividends in the Foreseeable Future.
The
Company has not paid cash dividends on its common stock since
inception. The intention of the Company is to retain earnings, if
any, for use in the business and does not anticipate paying any cash dividends
to stockholders in the foreseeable future.
The
Company’s Articles of Incorporation and By-laws Contain Certain Provisions that
May Be Barriers to a Takeover.
The
Company’s Articles of Incorporation and By-laws contain certain provisions which
may deter, discourage, or make it difficult to assume control of the Company by
another corporation or person through a tender offer, merger, proxy contest or
similar transaction or series of transactions. These provisions may
deter a future tender offer or other takeover attempt. Some
stockholders may believe such an offer to be in their best interest because it
may include a premium over the market
price of the common stock at the time. In addition, these provisions
may assist current management in retaining its position and place it in a better
position to resist changes which some stockholders may want to make if
dissatisfied with the conduct of the Company’s business.
Instability
And Volatility In The Financial Markets Could Have A Negative Impact On The
Company’s Business, Financial Condition, Results Of Operations And Cash
Flows.
During
recent months, there has been substantial volatility and a decline
in financial markets due at least in part to the deteriorating global
economic environment. In addition, there has been substantial uncertainty in the
capital markets and access to financing is uncertain. Moreover, customer
spending habits may be adversely affected by the current economic crisis.
These conditions could have an adverse effect on the Company’s industry and
business, including the Company’s financial condition, results of operations and
cash flows.
To the
extent that the Company does not generate sufficient cash from operations, it
may need to incur indebtedness to finance plans for growth. Recent turmoil in
the credit markets and the potential impact on the liquidity of major financial
institutions may have an adverse effect on the Company’s ability to fund its
business strategy through borrowings, under either existing or newly created
instruments in the public or private markets on terms that the Company believes
to be reasonable, if at all.
The Company Has Agreed to Indemnify
Its Officers and Directors From Liability.
Sections
78.7502 and 78.751 of the Nevada General Corporation Law allow the Company to
indemnify any person who is or was made a party to, or is or was threatened to
be made a party to, any pending, completed, or threatened action, suit
or
proceeding because he or she is or was a director, officer, employee or agent of
the Company or is or was serving at the Company’s request as a director,
officer, employee or agent of any corporation, partnership, joint venture, trust
or other enterprise. These provisions permit the Company to advance
expenses to an indemnified party in connection with defending any such
proceeding, upon receipt of an undertaking by the indemnified party to repay
those amounts if it is later determined that the party is not entitled to
indemnification. These provisions may also reduce the likelihood of
derivative litigation against directors and officers and discourage or deter
stockholders from suing directors or officers for breaches of their duties to
the Company, even though such an action, if successful, might otherwise benefit
the Company or its stockholders. In addition, to the extent that the
Company expends funds to indemnify directors and officers, funds will be
unavailable for operational purposes.
ITEM
1B. UNRESOLVED STAFF
COMMENTS
Not
Applicable
ITEM
2. PROPERTIES
The
corporate office of The Quigley Corporation is located at 621 Shady Retreat
Road, Doylestown, Pennsylvania. This property, with an area of
approximately 13,000 square feet, was purchased in November 1998 and refurbished
during 1999. The Company occupies warehouse space in Las Vegas,
Nevada at a current monthly cost of $2,772. This Nevada location has
a three-year lease that expires in July 2009. In addition to storage
facilities at the manufacturing subsidiary’s locations, the Company also stores
product in a number of additional warehouses in Pennsylvania with storage
charges based upon the quantities of product being stored.
The
manufacturing facilities of the Company are located in each of Elizabethtown and
Lebanon, Pennsylvania. The facilities were purchased effective
October 1, 2004. In total, the facilities have a total area of
approximately 73,000 square feet, combining both manufacturing and office
space. On February 2, 2009, the Company announced its intention
to close the Elizabethtown location which may result in the disposal of this
facility in the future.
The
Company believes that its existing facilities are adequate at this
time.
ITEM
3. LEGAL
PROCEEDINGS
TESAURO
AND ELEY, ET AL. VS. THE QUIGLEY CORPORATION
(CCP
of Phila., August Term 2000, No. 001011)
In
September 2000, the Company was sued by two individuals (Jason Tesauro and
Elizabeth Eley, both residents of Georgia), allegedly on behalf of a "nationwide
class" of "similarly situated individuals," in the Court of Common Pleas of
Philadelphia County, Pennsylvania. The Complaint further alleges that
the plaintiffs purchased certain Cold-Eeze products between August 1996, and
November 1999, based upon cable television, radio and internet advertisements,
which allegedly misrepresented the qualities and benefits of the Company's
products. The Complaint, as pleaded originally, requested an
unspecified amount of damages for violations of Pennsylvania's consumer
protection law, breach of implied warranty of merchantability and unjust
enrichment, as well as a judicial determination that the action be maintained as
a class action. In October 2000, the Company filed Preliminary
Objections to the Complaint seeking dismissal of the action. The
court sustained certain objections, thereby narrowing plaintiffs'
claims.
In May
2001, plaintiffs filed a motion to certify the putative class. The
Company opposed the motion. In November 2001, the court held a
hearing on plaintiffs' motion for class certification. In January
2002, the court denied in part and granted in part plaintiffs'
motion. The court denied plaintiffs' motion to certify a class based
on plaintiffs' claims under Pennsylvania's consumer protection law, under which
plaintiffs sought treble damages, effectively dismissing this cause of action;
however, the court certified a class based on plaintiffs' secondary breach of
implied warranty and unjust enrichment claims. In August, 2002, the
court issued an order adopting a form of Notice of Class Action to be published
nationally. Significantly, the form of Notice approved by the court
included a provision which limits the potential class members who may
potentially recover damages in this action to those persons who present a proof
of purchase of Cold-Eeze during the period August 1996 and November
1999.
Afterward,
a series of pre-trial motions were filed raising issues concerning trial
evidence and the court's jurisdiction over the subject matter of the
action. In March, 2005, the court held oral argument on these
motions.
Significantly,
on November 8, 2006, the Court entered an Order dismissing the case in its
entirety on the basis that the action was preempted by federal
law. The plaintiffs appealed the Court's decision in December, 2006
to the Superior Court of the Commonwealth of Pennsylvania. On
February 19, 2008, the Superior Court upheld defendant's appeal and remanded the
case to the Philadelphia County Court of Common Pleas for trial.
The case
commenced trial on February 2, 2009. On February 6, 2009, the jury
returned a verdict in favor of the Company on all counts. Plaintiffs
had to February 17, 2009, to file post-trial motions, the first step in the
appeal process. No post-trial motions were filed by the
plaintiffs. At this time the Company has no notice as to whether the
plaintiffs will attempt to perfect an appeal.
THE
QUIGLEY CORPORATION VS. JOHN C. GODFREY, ET AL.
(Bucks
Co. CCP, No. 04-07776)
In this
action, which was commenced in November 2004, the Company is seeking declaratory
and injunctive relief against John C. Godfrey, Nancy Jane Godfrey, and Godfrey
Science and Design, Inc. requesting injunctive relief regarding the Cold-Eeze
trade name and trademark; injunctive relief relating to the Cold-Eeze
formulations and manufacturing methods; injunctive relief for breach of the duty
of loyalty, and declaratory judgment pending the Company's payment of
commissions to defendants. The Company's Complaint is based in part
upon the Exclusive Representation and Distribution Agreement and the Consulting
Agreement (together the "Agreements") entered into between the defendants and
the Company. The Company terminated the Agreements for the
defendants' alleged material breaches of the Agreements. Defendants
have answered the complaint and asserted counterclaims against the Company
seeking remedies relative to the Agreements. The Company believes
that the defendants' counterclaims are without merit and is vigorously defending
those counterclaims and is prosecuting its action on its complaint.
The
discovery phase of pre-trial discovery is nearing
completion. Defendants moved for partial summary judgment, and the
Company filed a response and cross-motion for summary judgment. On
August 21, 2008, the court denied both motions for summary
judgment. The case has not been assigned to a trial calendar,
although it is possible that the case will be listed for trial in
2009.
At this time no prediction as to the
outcome of this action can be made.
NICODROPS,
INC. VS. QUIGLEY MANUFACTURING, INC.
On
January 30, 2006, Quigley Manufacturing, Inc., a wholly-owned subsidiary of The
Quigley Corporation, was put on notice of a claim by Nicodrops,
Inc. Nicodrops, Inc. has claimed that the packaging contained
incorrect expiration dates and caused it to lose sales through two (2)
retailers. The total alleged sales of Nicodrops was approximately
$250,000 and Nicodrops is claiming unspecified damages exceeding
$2,000,000.
No suit
has been filed. The Company is investigating this
claim. Based on its investigation to date, the Company believes the
claim is without merit. However, at this time no prediction can be
made as to the outcome of this case.
THE
QUIGLEY CORPORATION VS. WACHOVIA INSURANCE SERVICES, INC. AND FIRST
UNION
INSURANCE SERVICES AGENCY, INC.
The
Quigley Corporation instituted a Writ of Summons against Wachovia Insurance
Services, Inc. and First Union Insurance Services Agency, Inc. on December 8,
2005. The purpose of this suit was to maintain an action and toll the
statute of limitation against The Quigley Corporation's insurance broker who
failed to place excess limits coverage for the Company for the period from
November 29, 2003 until April 6, 2004. As a result of the defendant's
failure to place insurance and to notify the Company of its actions, certain
pending actions covered by the Company's underlying insurance at the present
time may result in certain cases presently being defended by insurance counsel
and the underlying insurance carrier to cause an exhaustion of the underlying
insurance for the policy periods ending November 29, 2004 and November 29,
2005. Any case in which an alleged action arose by the use of
COLD-EEZE Nasal Spray from November 29, 2003 to April 6, 2004 is not covered by
excess insurance.
The
Company's claim against Wachovia Insurance Services, Inc. and First Union
Insurance Services Agency, Inc. is for negligence and for equitable insurance
for these claims based on the Company's undertaking of certain attorneys' fees
and costs of settlement for claims that should have been covered by underlying
insurance placed by Wachovia Insurance Services, Inc.
At this
time no prediction can be made as to the outcome of any action against Wachovia
Insurance Services, Inc. and First Union Insurance Services Agency,
Inc.
TERMINATED
LEGAL PROCEEDINGS
CAROLYN SUNDERMEIER
VS. THE QUIGLEY CORPORATION
(Pa.
C.C.P., Bucks County, Docket No.: 07-01324-26-2)
On
February 16, 2007, plaintiff filed an action in the Court of Common Pleas of
Bucks County, Pennsylvania. The complaint was served on the Company
on February 20, 2007. The action alleges the plaintiff suffered
certain losses and injuries as a result of using the Company's nasal spray
product. Plaintiff's complaint consists of counts for negligence,
strict products liability (failure to warn), strict products liability
(defective design), breach of express and implied warranties, and violations
under the Pennsylvania Unfair Trade Practices and Consumer Protection Law and
other consumer protection statutes.
This action was recently settled at the
direction of the insurance carrier out of insurance proceeds.
MONIQUE FONTENOT DOYLE VS. THE QUIGLEY
CORPORATION
(U.S.D.C.,
W.D. La. Docket No.: 6:06CV1497)
On August
31, 2006, the plaintiff filed an action against the Company in the United States
District Court for the Western District of Louisiana (Lafayette-Opelousas
Division). The action alleges that the plaintiff suffered certain
losses and injuries as a result of the Company's nasal spray
product. Among the allegations of plaintiff are breach of express
warranties and damages pursuant to the Louisiana Products Liability
Act.
This case
was turned over to The Quigley Corporation for defense and settlement and it was
settled for less than the cost of defense after discovery was partially
completed. The cost of defense and the settlement remain claims
against Wachovia Insurance Services, Inc. and First Union Insurance Services
Agency, Inc. The Company's claim against Wachovia Insurance Services,
Inc. and First Union Services Agency, Inc. is for negligence and for equitable
insurance.
HOWARD POLSKI AND SHERYL POLSKI VS. THE
QUIGLEY CORPORATION, ET AL.
(U.S.D.C.,
D. Minn. Docket No.: 04-4199 PJS/JJG)
On August
12, 2004, plaintiffs filed an action against the Company in the District Court
for Hennepin County, Minnesota, which was not served until September 2,
2004. On September 17, 2004, the Company removed the case to the
United States District Court for the District of Minnesota. The
action alleges that plaintiffs suffered certain losses and injuries as a result
of the Company's nasal spray product. Among the allegations of
plaintiffs are negligence, products liability, breach of express and implied
warranties, and breach of the Minnesota Consumer Fraud Statute.
On
September 5, 2007, the Company obtained a judgment in its favor, as a matter of
law, and that decision was appealed to the Eighth Circuit Court of
Appeals. On August 13, 2008, the Eighth Circuit Court of Appeals
upheld the judgment in favor of the Company. The plaintiffs had until
December 3, 2008 to file a Petition for Allocatur to the Supreme Court of the
United States. No Petition for Allocatur was filed in this case and
the Company has a final judgment in its favor.
ITEM
4. SUBMISSION OF MATTERS TO A
VOTE OF SECURITY HOLDERS
None
PART II
ITEM
5. MARKET FOR REGISTRANT’S
COMMON EQUITY, RELATED STOCKHOLDER
MATTERS AND ISSUER PURCHASES
OF EQUITY SECURITIES
PERFORMANCE CHART
The
following graph reflects a five-year comparison, calculated on a dividend
reinvested basis, of the cumulative total stockholder return on the Common Stock
of the Company, a “peer group” index classified as drug related products by
Hemscott Group Ltd., (“Hemscott Group Index”) and the NASDAQ Market Index. The
comparisons utilize an investment of $100 on December 31, 2003 for the Company
and the comparative indices, which then measure the values for each group at
December 31 of each year presented. There can be no assurance that the Company’s
stock performance will continue with the same or similar trends depicted in the
following performance graph.
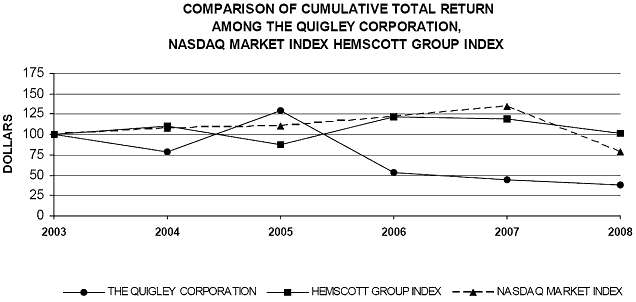
Market
Information
The
Company’s Common Stock, $.0005 par value, is currently traded on The NASDAQ
Global Market under the trading symbol “QGLY.” The price set forth in
the following table represents the high and low bid prices for the Company’s
Common Stock.
| Common Stock | ||||||||||||||||
|
2008
|
2007
|
|||||||||||||||
|
Quarter
Ended
|
High
|
Low
|
High
|
Low
|
||||||||||||
|
March
31
|
$ | 5.74 | $ | 4.17 | $ | 7.99 | $ | 5.09 | ||||||||
|
June
30
|
$ | 5.85 | $ | 4.54 | $ | 7.49 | $ | 4.55 | ||||||||
|
September
30
|
$ | 5.65 | $ | 4.58 | $ | 5.24 | $ | 2.92 | ||||||||
|
December
31
|
$ | 5.39 | $ | 2.85 | $ | 6.13 | $ | 3.75 | ||||||||
Such
quotations reflect inter-dealer prices, without mark-up, mark-down or commission
and may not represent actual transactions.
The
Company’s securities are traded on The NASDAQ Global Market and consequently
stock prices are available daily as generated by The NASDAQ Global Market
established quotation system.
Holders
As of
December 31, 2008, there were approximately 300 holders of record of the
Company’s Common Stock, including brokerage firms, clearing houses,
and/or depository firms holding the Company’s securities for their respective
clients. The exact number of beneficial owners of the Company’s
securities is not known but exceeds 400.
Dividends
The
Company has not declared, nor paid, any cash dividends on its Common
Stock. At this time the Company intends to retain its earnings to
finance future growth and maintain liquidity.
Warrants and
Options
In
addition to the Company’s outstanding Common Stock, there are, as of December
31, 2008, issued and outstanding Common Stock Purchase Warrants and Options that
are exercisable at the price-per-share stated and expire on the date indicated,
as follows:
|
Description
|
Number
|
Exercise
Price
|
Expiration
Date
|
||||||
|
Option
Plan
|
331,000 | $ | 5.1250 |
April
6, 2009
|
|||||
|
Option
Plan
|
160,500 | $ | 0.8125 |
December
20, 2010
|
|||||
|
Option
Plan
|
153,500 | $ | 1.2600 |
December
10, 2011
|
|||||
|
Option
Plan
|
291,250 | $ | 5.1900 |
July
30, 2012
|
|||||
|
Option
Plan
|
42,500 | $ | 5.4900 |
December
17, 2012
|
|||||
|
Option
Plan
|
370,500 | $ | 8.1100 |
October
29, 2013
|
|||||
|
Option
Plan
|
435,500 | $ | 9.5000 |
October
26, 2014
|
|||||
|
Option
Plan
|
483,500 | $ | 13.8000 |
December
11,
2015
|
|||||
At
December 31, 2008, there were 2,268,250 unexercised and vested options of the
Company’s Common Stock available for exercise.
Securities Authorized Under
Equity Compensation
The
following table sets forth certain information regarding stock option and
warrant grants made to employees, directors and consultants:
SECURITIES AUTHORIZED FOR ISSUANCE
UNDER EQUITY COMPENSATION PLANS
|
Plan
Category
|
Number
of Securities to be Issued Upon Exercise of Outstanding Options &
Warrants
(A)
|
Weighted
Average Exercise Price of Outstanding Options & Warrants
(B)
|
Number
of Securities Remaining Available for Future Issuance Under Equity
Compensation Plans (Excluding Securities Reflected in Column
A)
( C
)
|
||||
|
Equity
Plans Approved by Security Holders (1)
|
2,268,250
|
$7.76
|
1,753,750
|
||||
|
(1)
|
An
incentive stock option plan was instituted in 1997, (the “1997 Stock
Option Plan”) and approved by the stockholders in 1998. Options pursuant
to the 1997 Stock Option Plan have been granted to directors, executive
officers, and employees.
|
ITEM
6. SELECTED FINANCIAL
DATA
The
following table sets forth the selected financial data of the Company for and at
the end of the years ended December 31, 2008, 2007, 2006, 2005 and
2004.
The data
presented below should be read in conjunction with “Management’s Discussion and
Analysis of Financial Condition and Results of Operation” and the Company’s
financial statements and notes thereto appearing elsewhere herein.
|
(Amounts
in thousands, except per
share data)
|
Year Ended
December
31,
2008
|
Year Ended
December
31,
2007
|
Year Ended
December
31,
2006
|
Year Ended
December
31,
2005
|
Year Ended
December
31,
2004
|
|||||||||||||||
|
Statement
of Income Data:
|
||||||||||||||||||||
|
Net
sales
|
$ | 20,507 | $ | 28,242 | $ | 26,850 | $ | 33,185 | $ | 23,587 | ||||||||||
|
Gross
profit
|
$ | 11,413 | $ | 18,556 | $ | 17,545 | $ | 21,301 | $ | 13,546 | ||||||||||
|
(Loss)
income - continuing operations
|
$ | (6,410 | ) | $ | (1,856 | ) | $ | (547 | ) | $ | 2,339 | $ | (1,060 | ) | ||||||
|
Income
(loss) - discontinued operations (1)
|
$ | 876 | $ | (602 | ) | $ | (1,201 | ) | $ | 878 | $ | 1,513 | ||||||||
|
Net
(loss) income
|
$ | (5,534 | ) | $ | (2,458 | ) | $ | (1,748 | ) | $ | 3,217 | $ | 453 | |||||||
|
Basic
(loss) earnings per share:
Continuing
operations
|
$ | (0.50 | ) | $ | (0.14 | ) | $ | (0.04 | ) | $ | 0.20 | $ | (0.09 | ) | ||||||
|
Discontinued
operations
|
$ | 0.07 | $ | (0.05 | ) | $ | (0.10 | ) | $ | 0.08 | $ | 0.13 | ||||||||
|
Net
(loss) income
|
$ | (0.43 | ) | $ | (0.19 | ) | $ | (0.14 | ) | $ | 0.28 | $ | 0.04 | |||||||
|
Diluted
(loss) earnings per share:
Continuing
operations
|
$ | (0.50 | ) | $ | (0.14 | ) | $ | (0.04 | ) | $ | 0.17 | $ | (0.07 | ) | ||||||
|
Discontinued
operations
|
$ | 0.07 | $ | (0.05 | ) | $ | (0.10 | ) | $ | 0.07 | $ | 0.10 | ||||||||
|
Net
income (loss)
|
$ | (0.43 | ) | $ | (0.19 | ) | $ | (0.14 | ) | $ | 0.24 | $ | 0.03 | |||||||
|
Weighted
average shares outstanding:
|
||||||||||||||||||||
|
Basic
|
12,878 | 12,729 | 12,245 | 11,661 | 11,541 | |||||||||||||||
|
Diluted
|
12,878 | 12,729 | 12,245 | 13,299 | 14,449 | |||||||||||||||
|
As
of
December
31,
2008
|
As
of
December
31,
2007
|
As
of
December
31,
2006
|
As
of
December
31,
2005
|
As
of
December
31,
2004
|
||||||||||||||||
|
Balance
Sheet Data:
|
||||||||||||||||||||
|
Working
capital
|
$ | 14,072 | $ | 18,578 | $ | 20,541 | $ | 20,682 | $ | 17,853 | ||||||||||
|
Total
assets
|
$ | 24,369 | $ | 33,502 | $ | 34,845 | $ | 35,976 | $ | 31,530 | ||||||||||
|
Debt
|
$ | - | $ | - | $ | - | $ | 1,464 | $ | 2,893 | ||||||||||
|
Stockholders’
equity
|
$ | 17,774 | $ | 23,244 | $ | 25,529 | $ | 25,320 | $ | 21,902 | ||||||||||
(1) On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc. (See
Item 7, “Management’s Discussion and Analysis of Financial Condition and Results
of Operations” and Note 3 “Discontinued Operations” for additional
information.) The sale of this segment has been treated as discontinued
operations and all periods presented have been reclassified.
|
|
ITEM 7.
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
|
Overview
The
Company, headquartered in Doylestown, Pennsylvania, is a leading manufacturer,
marketer and distributor of a diversified range of homeopathic and health
products which comprise the Cold Remedy and Contract Manufacturing
segments. The Company is also involved in the research and
development of potential natural base health products, including, but not
limited to, prescription medicines along with supplements and cosmeceuticals for
human and veterinary use, which comprise the Ethical Pharmaceutical
segment.
The
Company’s primary business is the manufacture and distribution of cold remedy
products to the consumer through the over-the-counter
marketplace. One of the Company’s key products in its Cold Remedy
segment is Cold-EezeÒ,
a zinc gluconate glycine product proven in two double-blind clinical studies to
reduce the duration and severity of the common cold symptoms by nearly half.
Cold-Eeze is an established product in the health care and cold remedy
market.
Effective
October 1, 2004, the Company acquired substantially all of the assets of JoEl,
Inc., the previous manufacturer of the Cold-Eeze lozenge
product. This manufacturing entity, now called QMI, a wholly-owned
subsidiary of the Company, will continue to produce lozenge product along with
performing such operational tasks as warehousing and shipping the Company’s
Cold-Eeze products. In addition, QMI, which is an FDA approved
facility, has produced a variety of hard and organic candy for sale to third
party customers in addition to performing contract manufacturing activities for
non-related entities. On February 2, 2009, the Company announced its
intention to close the Elizabethtown location of QMI and discontinue the hard
candy business resulting in the consolidation of manufacturing operations at the
Lebanon location. This consolidation will have no impact on the
production or distribution of the Cold-EezeÒ brand of cold remedy
products.
The
Company’s Cold Remedy segment reported a sales decrease in 2008 compared to
2007. This decrease may be attributable to continued customer review
of inventory levels and product mix particularly in light of current market and
economic conditions including higher than normal product returns. The
cough/cold segment has been adversely affected in the past two cold seasons by
the least incidence of colds by consumers in the last several
years. The 2008 sales activity reflects the market wide decrease in
cold remedy product consumption as supported by recent Information Resources
Inc. (“IRI”) data, which was consistent throughout 2008. Cold-Eeze continues to
compete with new products entering the category despite many of these products
being without any evidence of clinical effectiveness, unlike Cold-Eeze which has
been clinically proven to treat the common cold.
In 2008,
the margin of the Cold Remedy segment was adversely affected as a result of
decreased sales and higher than normal products returns along with product
obsolescence costs. The consolidated margin was also impacted by
reduced production at the manufacturing facilities resulting in a negative
impact to margin. The 2008 margin was supported as a result of the
discontinuation in May 2007 of royalty costs associated with the developer of
Cold-Eeze along with a price increase of Cold-Eeze to the trade in July 2007. In
2008, the Company recognized an impairment charge of $300,000 due to adverse
profit margins related to the hard candy business of QMI with such expense
reflected in cost of sales. In February 2009, the Company announced
plans to discontinue its hard candy business resulting in the closure of the
Elizabethtown, Pennsylvania, manufacturing location in 2009 and consolidate its
manufacturing capabilities to one location in order to improve manufacturing
efficiencies. The facility located in Lebanon, Pennsylvania,
currently manufactures the Cold-Eeze lozenge product and will continue to do so
along with warehousing and distributing the Company’s range of cold remedy
products.
In
January 2001, the Company formed an Ethical Pharmaceutical segment, Pharma, that
is under the direction of its Executive Vice President and Chairman of its
Medical Advisory Committee. Pharma was formed for the purpose of developing
potential natural base health products, including, but not limited to,
prescription medicines along with supplements and cosmeceuticals for human and
veterinary use. Pharma is currently undergoing research and development activity
in compliance with regulatory requirements. The Company is in the initial stages
of what may be a lengthy process to develop these patent applications into
commercial products. The Company continues to invest significantly with ongoing
research and development activities of this segment.
On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc., whose
major shareholder is Mr. Kevin P. Brogan, the current president of
Darius. The terms of the agreement included a cash purchase price of
$1,000,000 by InnerLight Holdings, Inc., for the stock of Darius and its
subsidiaries without guarantees, warranties or
indemnifications. Darius, through its wholly-owned subsidiary,
Innerlight Inc., constituted the Health and Wellness segment of the
Company. The divestiture of Darius will provide clarity to the
Company’s strategic plan to focus its future endeavors in a pharmaceutical
entity with OTC products and a pipeline of potential formulations that may lead
to prescription and other medicinal products. The sale of this Health
and Wellness segment has been treated as discontinued operations and all periods
presented have been reclassified.
Future
revenues, costs, margins, and profits will continue to be influenced by the
Company’s ability to maintain its manufacturing availability and capacity
together with its marketing and distribution capabilities and the requirements
associated with the development of Pharma’s potential prescription drugs and
other medicinal products in order to continue to compete on a national and
international level. The business development of the Company is
dependent on continued conformity with government regulations, a reliable
information technology system capable of supporting continued growth and
continued reliable sources for product and materials to satisfy consumer
demand.
Effect of
Recent Accounting Pronouncements
In
September 2006, the Financial Accounting Standards Board (FASB) issued
Statement of Financial Accounting Standards No. 157, “Fair Value Measurements”
(“SFAS 157”). SFAS 157 defines fair value, establishes a framework for
measuring fair value in generally accepted accounting principles (GAAP) and
expands disclosures about fair value measurements. SFAS 157
is effective for fiscal years beginning after November 15, 2007 and interim
periods within those fiscal years. The adoption of this standard has not
had a significant impact on the Company’s consolidated financial position,
results of operations or cash flows.
In
February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial
Assets and Financial Liabilities”, including an amendment of FASB No. 115
("FAS 159"). The Statement permits companies to choose to measure many financial
instruments and certain other items at fair value in order to mitigate
volatility in reported earnings caused by measuring related assets and
liabilities differently without having to apply complex hedge accounting
provisions. FAS 159 is effective for the Company beginning January 1, 2008. The
adoption of this standard has not had a significant impact on the Company’s
consolidated financial position, results of operations or cash
flows.
In
December 2007, the FASB issued Statement of Financial Accounting Standard
No. 160, “Noncontrolling
Interests in Consolidated Financial Statements — an amendment of ARB
No. 51” (“FAS 160”). FAS 160 establishes accounting and
reporting standards for the non-controlling interest in a subsidiary and for the
retained interest and gain or loss when a subsidiary is deconsolidated. This
statement is effective for financial statements issued for fiscal years
beginning on or after December 15, 2008 with earlier adoption prohibited.
The adoption of this standard is not expected to have a significant impact on
the Company’s consolidated financial position, results of operations or cash
flows.
In
December 2007, the FASB issued SFAS No. 141R, "Business Combinations,"
(“SFAS 141R”) which establishes principles and requirements for how an
acquirer recognizes and measures in its financial statements the identifiable
assets acquired, the liabilities assumed and any non-controlling interest in the
acquiree. SFAS 141R also establishes disclosure requirements to enable the
evaluation of the nature and financial effects of the business combination. SFAS
141R applies prospectively to business combinations for which the acquisition
date is on or after the beginning of the first annual reporting period beginning
on or after December 15, 2008, and interim periods
within those fiscal years.
The adoption of this standard will not have any impact on the Company’s
consolidated financial position, results of operations or cash
flows.
Critical Accounting
Estimates
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States requires management to make estimates
and assumptions that affect the reported amounts of assets and liabilities and
disclosure of contingent liabilities at the dates of the financial statements
and the reported amounts of revenues and expenses during the reporting
periods. Actual results could differ from those
estimates.
The
Company is organized into three different but related business segments, Cold
Remedy, Contract Manufacturing and Ethical Pharmaceutical. When providing for
the appropriate sales returns, allowances, cash discounts and cooperative
incentive promotion costs, each segment applies a uniform and consistent method
for making certain assumptions for estimating these provisions that are
applicable to that specific segment. Traditionally, these provisions are not
material to net income in the Contract Manufacturing segment. The Ethical
Pharmaceutical segment does not have any revenues.
The
primary product in the Cold Remedy segment, Cold-EezeÒ, has been clinically
proven in two double-blind studies to reduce the severity and duration of common
cold symptoms. Accordingly, factors considered in estimating the appropriate
sales returns and allowances for this product include it being: a unique product
with limited competitors; competitively priced; promoted; unaffected for
remaining shelf life as there is no expiration date; monitored for inventory
levels at major customers and third-party consumption data, such as
IRI.
At
December 31, 2008 and 2007 the Company included reductions to accounts
receivable for sales returns and allowances of $1,427,000 and $296,000,
respectively, and cash discounts of $150,000 and $169,000, respectively.
Additionally, current liabilities at December 31, 2008 and 2007 include
$1,058,962 and $1,137,650, respectively for cooperative incentive promotion
costs.
The
roll-forward of the sales returns and allowance reserve ending at December 31 is
as follows:
|
Account
– Sales Returns & Allowances
|
2008
|
2007
|
||||||
|
Beginning
balance
|
$ | 295,606 | $ | 473,176 | ||||
|
Provision
made for future charges relative to sales for each period
presented
|
2,354,346 | 1,104,161 | ||||||
|
Current
provision related to discontinuation of Cold-EezeÒ nasal
spray
|
- | - | ||||||
|
Actual
returns & allowances recorded in the current period
presented
|
(1,222,907 | ) | (1,281,731 | ) | ||||
|
Ending
balance
|
$ | 1,427,045 | $ | 295,606 | ||||
The
increase in the 2008 provision was principally due to non-routine returns of
obsolete product and product mix realignment by certain of our
customers. Also, the Company applies specific limits on product
returns from customers, and evaluates return requests from customers relative to
the Cold Remedy segment.
Management
believes there are no material charges to net income in the current period,
related to sales from a prior period.
Revenue
Provisions
to reserves to reduce revenues for cold remedy products that do not have an
expiration date, include the use of estimates, which are applied or matched to
the current sales for the period presented. These estimates are based on
specific customer tracking and an overall historical experience to obtain an
effective applicable rate, which is tested on an annual basis and reviewed
quarterly to ascertain the most applicable effective rate. Additionally, the
monitoring of current occurrences, developments by customer, market conditions
and any other occurrences that could affect the expected provisions relative to
net sales for the period presented are also performed.
A one
percent deviation for these consolidated reserve provisions for the years ended
December 31, 2008, 2007 and 2006 would affect net sales by approximately
$276,000, $348,000 and $318,000, respectively. A one percent deviation for
cooperative incentive promotions reserve provisions for the years ended December
31, 2008, 2007 and 2006 could affect net sales by approximately $252,000,
$323,000 and $298,000, respectively.
Income
Taxes
The
Company has recorded a valuation allowance against its net deferred tax
assets. Management believes that this allowance is required due to
the uncertainty of realizing these tax benefits in the future. The
uncertainty arises because the Company may incur substantial research and
development costs in its Ethical Pharmaceutical segment.
Results of
Operations
Year ended December 31, 2008
compared with same period 2007
Net sales
for 2008 were $20,506,612 compared to $28,241,502 for 2007, reflecting a
decrease of $7,734,890 or 27.4% in 2008. Revenues, by segment, for
2008 were Cold Remedy, $18,185,510 and Contract Manufacturing, $2,321,102; as
compared to 2007, when the revenues for each respective segment were $25,730,016
and $2,511,486.
The Cold
Remedy segment reported a sales decrease in 2008 of $7,544,506 or
29.3%. This decrease may be attributable to continued customer review
of inventory levels and product mix particularly in light of current market and
economic conditions including higher than normal product returns. The
cough/cold segment has been adversely affected in the past two cold seasons by
the least incidence of colds by consumers in the last several
years. The 2008 sales activity reflects the market wide decrease in
cold remedy product consumption as supported by recent IRI data, which was
consistent throughout 2008. Cold-Eeze continues to compete with new products
entering the category despite many of these products being without any evidence
of clinical effectiveness, unlike Cold-Eeze which has been clinically proven to
treat the common cold.
The
Company is continuing to strongly support Cold-Eeze as a clinically proven cold
remedy product through in-store promotion, media advertising and coupon
programs.
The
Contract Manufacturing segment refers to the third party sales generated by
QMI. In addition to the manufacture of the Cold-EezeÒ product, QMI also
manufactures a variety of hard and organic candies under its own brand names
along with other products on a contract manufacturing basis for other
customers. Sales for this segment in 2008 decreased by $190,384 or
7.6%.
Consolidated
cost of sales from continuing operations for 2008 as a percentage of net sales
was 44.3%, compared to 34.3% for 2007. The cost of sales percentage
for the Cold Remedy segment increased in 2008 by 5.4% primarily due to higher
than normal product returns along with product obsolescence costs in 2008, with
these two items increasing 2008 cold remedy costs of sales by 6.4% over
2007. The 2007 cost of sales also reflects a royalty charge which
amounted to 1.2% of sales with no such expense in 2008 due to the expiration of
the royalty agreement.
The 2008
gross margin was reduced due to decreased cold remedy product sales along with
increased returns and costs of product obsolescence. The 2008 margin
was also impacted by reduced production in the Contract Manufacturing segment.
In 2008, the Company recognized an impairment charge of $300,000 due to adverse
profit margins related to the hard candy business of Quigley Manufacturing Inc.
with such expense reflected in cost of sales. In February 2009, the
Company announced plans to discontinue its hard candy business resulting in the
closure of the Elizabethtown, Pennsylvania, manufacturing location in 2009 and
consolidate its manufacturing capabilities to one location in order to improve
manufacturing efficiencies. The facility located in Lebanon,
Pennsylvania, currently manufactures the Cold-Eeze lozenge product and will
continue to do so along with warehousing and distributing the Company’s range of
cold remedy products.
Selling,
marketing and administrative expenses for 2008 were $13,901,159 compared to
$14,621,612 in 2007. The
decrease in 2008 was primarily due to increased
outside advertising, marketing and promotional costs of $1,548,937, primarily
due to increased media advertising; decreased sales
brokerage commission costs of $252,000 due to less 2008
cold remedy sales; payroll costs decreased by $1,100,000, mainly due
to decreased 2008 general payroll and bonus costs; legal costs decreased by
$455,000 and stock promotion decreased by $173,000. Selling,
marketing and administrative expenses, by segment, in 2008 were Cold Remedy
$11,662,725; Pharma, $718,076; and Contract Manufacturing, $1,520,358; as
compared to expenses in 2007 of $12,387,758, $602,409 and $1,631,445,
respectively.
Research
and development costs for 2008 and 2007 were $4,241,724 and $6,482,485,
respectively. Principally, the decrease in research and development
expenditure was the result of decreased Pharma study costs of approximately
$2,200,000 in 2008.
During
2008, the Company’s major operating expenses of salaries, brokerage commissions,
promotion, advertising, and legal costs accounted for approximately $12,412,984
(68.4%) of the total operating expenses of $18,142,883, a decrease of 3.0% over
the 2007 amount of $12,790,768 (60.6%) of total operating expenses of
$21,104,097, largely the result of increased advertising and promotion,
decreased brokers commission, decreased legal costs and decreased payroll costs
in 2008.
Total
assets of the Company at December 31, 2008 and 2007 were $24,368,631 and
$33,501,921, respectively. Working capital decreased by $4,505,948 to
$14,071,676 at December 31, 2008. The primary influences on working
capital during 2008 were: the decrease in cash balances; decreased accounts
receivable balances; decreased inventory on hand; decreased other liabilities
and decreased advertising payable balances due to variations in advertising
scheduling and strategies between years and related seasonal
factors.
On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc. Darius,
through its wholly-owned subsidiary, Innerlight Inc., constituted the Health and
Wellness segment of the Company. The divestiture of Darius will
provide clarity to the Company’s strategic plan to focus its future endeavors in
a pharmaceutical entity with OTC products and a pipeline of potential
formulations that may lead to prescription and other medicinal
products. The sale of this segment has been treated as discontinued
operations and all periods presented have been reclassified.
Year ended December 31, 2007
compared with same period 2006
Net sales
for 2007 were $28,241,502 compared to $26,850,030 for 2006, reflecting an
increase of 5.2% in 2007. Revenues, by segment, for 2007 were Cold
Remedy, $25,730,016 and Contract Manufacturing, $2,511,486; as compared to 2006,
when the revenues for each respective segment were $24,815,851 and
$2,034,179.
The Cold
Remedy segment reported a sales increase in 2007 of $914,165 or 3.7%. This
increase reflects the launch of the Organix™ and Immune products in the third
quarter 2007, contributing combined net sales of $2,017,316. Additionally, the
Cold-Eeze price increase to the trade on July 1, 2007 contributed additional net
sales amount of approximately $2,250,000. The 2007 sales activity indicates
reduced unit sales of Cold-Eeze to retail which is reflective of IRI reports
indicating a substantial decrease in unit consumption of Cold-Eeze in 2007, both
in the fourth quarter and over the twelve month
period. Available IRI reports indicate that the 2007 cough/cold
season had the lowest reported incidence of the common cold in over eight years,
a factor which had consequences across the cough/cold
category. Revenues of this segment were also negatively impacted by
the reduction in warehouse and retail inventory levels of several key retail
outlets. New competitor products continue to enter into the retail
arena and vie for visibility in an already congested category. Unlike
Cold-Eeze, which is clinically proven to treat the common cold, many of these
new products are without any evidence of clinical effectiveness. The
Company is continuing to strongly support Cold-Eeze as a clinically proven cold
remedy product through in-store promotion, media advertising and the
introduction of new flavors.
The
Contract Manufacturing segment refers to the third party sales generated by
QMI. In addition to the manufacture of the Cold-EezeÒ product, QMI also
manufactures a variety of hard and organic candies under its own brand names
along with other products on a contract manufacturing basis for other
customers. Sales for this segment in 2007 increased by $477,307 or
23.5%.
Cost of
sales from continuing operations for 2007 as a percentage of net sales was
34.3%, compared to 34.7% for 2006. The cost of sales percentage for
the Cold Remedy segment decreased in 2007 by 1.6% primarily due to the impact of
the discontinuation of the Company’s royalty obligations to the developers in
May 2007, a favorable effect of 3.4% in 2007, the launch of the two new products
and the impact of the Cold-Eeze price increase resulted in a combined increase
in cost of 0.7% and the adverse impact of the coupon programs on cost of goods
was 1.4%.
The 2007
and 2006 consolidated cost of sales were both favorably impacted as a result of
the consolidation effects of the manufacturing facility as it relates to
Cold-EezeÒ. These gross
profit gains of the Cold Remedy segment were mitigated by substantially lower
gross profit margins for the Contract Manufacturing segment, which is
significantly lower than the other operating segments.
Selling,
marketing and administrative expenses for 2007 were $14,621,612 compared to
$14,921,437 in 2006. The
decrease in 2007 was primarily due to decreased
outside advertising product marketing and promotional costs of $2,054,000,
primarily due to a reduction in media advertising with a change to various
coupon programs the costs of which are accounted for as a reduction from
sales. Sales brokerage commission costs increased by
$275,000 due to increased 2007 cold remedy sales; payroll costs
increased by $1,157,000, mainly due to increased 2007 bonuses; legal costs
increased by $127,000, insurance costs decreased by $419,000, stock promotion
increased by $184,000. Selling, marketing and administrative
expenses, by segment, in 2007 were Cold Remedy $12,387,758; Pharma $602,409; and
Contract Manufacturing $1,631,445; as compared to 2006 of $12,605,400, $743,465
and $1,572,572, respectively.
Research
and development costs for 2007 and 2006 were $6,482,485 and $3,787,498,
respectively. Principally, the increase in research and development
expenditure was the result of increased Pharma study costs of approximately
$2,772,000 in 2007.
During
2007, the Company’s major operating expenses of salaries, brokerage commissions,
promotion, advertising, and legal costs accounted for approximately $12,790,768
(60.6%) of the total operating expenses of $21,104,097, a decrease of 2.0% over
the 2006 amount of $13,054,170 (69.8%) of total operating expenses of
$18,708,935, largely the result of decreased advertising, increased brokers
commission and increased payroll costs in 2007.
Total
assets of the Company at December 31, 2007 and 2006 were $33,501,921 and
$34,845,034, respectively. Working capital decreased by $1,963,649 to
$18,577,624 at December 31, 2007. The primary influences on working
capital during 2007 were: the decrease in cash balances, increased inventory on
hand; increased accrued royalties and sales commissions as a result
of litigation between the Company and the developer of Cold-Eeze,
increased other liabilities and decreased advertising payable balances due to
variations in advertising scheduling and strategies between years and related
seasonal factors.
Material Commitments and
Significant Agreements
Effective
October 1, 2004, the Company acquired certain assets and assumed certain
liabilities of JoEl, Inc., the sole manufacturer of the Cold-EezeÒ lozenge
product. As part of the acquisition, the Company entered into a loan
obligation in the amount of $3.0 million payable to PNC Bank, N.A.
The loan was collateralized by mortgages on real property located in each of
Lebanon, Pennsylvania and Elizabethtown, Pennsylvania and was used to finance
the majority of the cash portion of the purchase price. The Company
could elect interest rate options of
either the Prime Rate or LIBOR plus 200 basis points. The loan was
payable in eighty-four equal monthly principal payments of $35,714 commencing
November 1, 2004, and such amounts payable were reflected in the consolidated
balance sheet as current portion of
long-term debt amounting to $428,571 and
long-term debt amounting to $1,035,715 at
December 31, 2005. The loan was completely
repaid in 2006. During the duration of the loan, the Company was in
compliance with all related loan covenants.
With the
exception of the Company’s Cold-EezeÒ brand lozenge products and
QMI’s sales to third party customers, the Company’s products are manufactured by
outside sources. The Company has agreements in place with these
manufacturers, which ensure a reliable source of product for the
future.
The
Company has agreements in place with independent brokers whose function is to
represent the Company’s Cold-EezeÒ products, in a product
sales and promotion capacity, throughout the United States and
internationally. The brokers are remunerated through a commission
structure, based on a percentage of sales collected, less certain
deductions.
The
Company has maintained a separate representation and distribution agreement
relating to the development of the zinc gluconate glycine product
formulation. In return for exclusive distribution rights, the Company
must pay the developer a 3% royalty and a 2% consulting fee based on sales
collected, less certain deductions, throughout the term of this agreement, which
expired in May 2007. However, the Company and the developer are in
litigation and as such, no potential offset for these fees from such litigation
has been recorded. A founder’s commission totaling 5%, on sales
collected, less certain deductions, has been paid to two of the officers of the
Company, who are also directors and stockholders of the Company, and whose
agreements expired in May 2005. The expenses for the respective
periods relating to such agreements amounted to zero, $293,266 and $1,153,354
for the year ended December 31, 2008, 2007 and 2006,
respectively. Amounts accrued for these expenses at December
31, 2008 and 2007 were $3,524,031 on both dates.
On
February 24, 2009, The Quigley Corporation announced that it had signed a
license with assignment of ownership agreement for its patented formulation
QR-340 developed by its wholly owned subsidiary, Pharma. The compound has been
clinically tested and shown to improve the appearance of scars in a comparative
study. The Agreement is with Levlad, LLC/Natures Gate, a manufacturer and
marketer of personal care products based on botanicals.
The
general terms of the agreement allow the assignee to further refine, develop and
commercialize the product with exclusivity and eventual full ownership of the
patent within five years, beginning January 2009. The agreement is based on
required royalty payments totaling $1.1 million to The Quigley Corporation over
the time period. Under the terms of the agreement, if the minimum payments and
terms are not met within the five year period, the Company retains full rights
and ownership of the property. However, Levlad can continue to pay per unit
royalties beyond five years for a non-exclusive license.
Certain
operating leases for office and warehouse space maintained by the Company
resulted in rent expense for the years ended December 31, 2008, 2007 and 2006,
of $53,200, $68,436, and $60,735, respectively. The future minimum lease
obligations under these operating leases are approximately $19,400.
Liquidity and Capital
Resources
The
Company had working capital of $14,071,676 and $18,577,624 at December 31, 2008
and 2007, respectively. Changes in working capital overall have been primarily
due to the following items: cash balances decreased by $3,176,750; account
receivable balances, net, decreased by $2,125,019 due to decreased cold remedy
sales and effective collection practices; inventory decreased by $1,134,510
primarily due to reduced cold remedy sales and obsolescence provisions, other
current liabilities decreased by $1,739,074 primarily due to reduced payroll,
legal and research and development accruals; accrued royalties and sales
commissions decreased by $67,768 largely due to decreased cold remedy sales.
Total cash balances at December 31, 2008 were $11,956,796 compared to
$15,133,546 at December 31, 2007.
Management
believes that its strategy to establish Cold-EezeÒ as a recognized brand
name, its broader range of products, adequate manufacturing capacity, together
with its current working capital, should provide an internal source of capital
to fund the Company’s normal business operations. The operations of
the Company contribute to the current research and development expenditures of
the Ethical Pharmaceutical segment. In addition to the funding
from operations, the Company may in the short and long term raise capital
through the issuance of equity securities or secure other financing resources to
support such research. As research progresses on certain
formulations, expenditures of the Pharma segment will require substantial
financial support and would necessitate the consideration of other approaches
such as licensing or partnership arrangements that meet the Company’s long term
goals and objectives. Ultimately, should internal working capital or
internal funding be insufficient, there is no guarantee that other financing
resources will become available, thereby deferring future growth and development
of certain formulations.
On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc., whose
major shareholder is Mr. Kevin P.
Brogan, the current president of Darius. The terms
of the agreement include a cash purchase price
of $1,000,000 by InnerLight
Holdings, Inc. for the stock of Darius and its subsidiaries without guarantees,
warranties or indemnifications. Darius markets health and wellness
products through its wholly-owned subsidiary, Innerlight Inc., which constituted
the Health and Wellness segment of the Company. Losses from this
segment in recent times have reduced the resources available for the research
and development activities of the Pharma segment. Additionally, the
divestiture of Darius will provide clarity to the Company’s strategic plan to
focus its future endeavors in a pharmaceutical entity with OTC products and a
pipeline of potential formulations that may lead to prescription and other
medicinal products. The sale of this segment has been treated as
discontinued operations and all periods presented have been
reclassified.
Management
is not aware of any trends, events or uncertainties that have or are reasonably
likely to have a material negative impact upon the Company’s (a) short-term or
long-term liquidity, or (b) net sales or income from continuing
operations. Any challenge to the Company’s patent rights could have a
material adverse effect on future liquidity of the Company; however, the Company
is not aware of any condition that would make such an event
probable.
Management
believes that cash generated from operations, along with its current cash
balances, will be sufficient to finance working capital and capital expenditure
requirements for at least the next year.
Contractual
Obligations
The
Company’s future contractual obligations and commitments at December 31, 2008
consist of the following:
|
Payment Due by
Period
|
||||||||||||||||||||
|
Contractual
Obligations
|
Total
|
Less
than
1
year
|
1-3
years
|
4-5
years
|
More
than
5
years
|
|||||||||||||||
|
Operating
Lease Obligations
|
$ | 19,406 | $ | 19,406 | $ | - | $ | - | $ | - | ||||||||||
|
Purchase
Obligations
|
3,347,000 | 1,355,000 | 1,992,000 | - | - | |||||||||||||||
|
Research
and Development
|
442,000 | 442,000 | - | - | - | |||||||||||||||
|
Advertising
|
1,920,173 | 1,920,173 | - | - | - | |||||||||||||||
|
Total
Contractual Obligations
|
$ | 5,728,579 | $ | 3,736,579 | $ | 1,992,000 | $ | - | $ | - | ||||||||||
Off-Balance
Sheet Arrangements
It is not
the Company's usual business practice to enter into off-balance sheet
arrangements such as guarantees on loans and financial commitments and retained
interests in assets transferred to an unconsolidated entity for securitization
purposes. Consequently, the Company has no off-balance sheet
arrangements that have, or are reasonably likely to have, a material current or
future effect on its financial condition, changes in financial condition,
revenues or expenses, results of operations, liquidity, capital expenditures or
capital resources.
Impact of
Inflation
The
Company is subject to normal inflationary trends and anticipates that any
increased costs would be passed on to its customers.
ITEM
7A. QUANTITATIVE AND QUALITATIVE
DISCLOSURES ABOUT MARKET RISK
The
Company's operations are not subject to risks of material foreign currency
fluctuations, nor does it use derivative financial instruments in its investment
practices. The Company places its marketable investments in instruments that
meet high credit quality standards. The Company does not expect material losses
with respect to its investment portfolio or exposure to market risks associated
with interest rates. The impact on the Company's results of one percentage point
change in short-term interest rates would not have a material impact on the
Company’s future earnings, fair value, or cash flows related to investments in
cash equivalents or interest-earning marketable securities.
Current
economic conditions may cause a decline in business and consumer spending which
could adversely affect the Company’s business and financial performance
including the collection of accounts receivables, realization of inventory and
recoverability of assets. In addition, the Company’s business and
financial performance may be adversely affected by current and future economic
conditions, including due to a reduction in the availability of credit,
financial market volatility and recession.
ITEM 8. FINANCIAL STATEMENTS AND
SUPPLEMENTARY DATA
|
INDEX
TO CONSOLIDATED FINANCIAL STATEMENTS
|
||
|
Page
|
||
|
Balance
Sheets as of December 31, 2008 and 2007
|
F-1
|
|
|
Statements
of Operations for the years ended December 31, 2008, 2007, and
2006
|
F-2
|
|
|
Statements
of Stockholders’ Equity for the years ended December 31, 2008, 2007, and
2006
|
F-3
|
|
|
Statements
of Cash Flows for the years ended December 31, 2008, 2007, and
2006
|
F-4
|
|
|
Notes
to Financial Statements
|
F-5
to F-21
|
|
|
Responsibility
for Financial Statements
|
F-22
|
|
|
Report
of Independent Registered Public Accounting Firm
Amper, Politziner
& Mattia, LLP
|
F-23
|
THE
QUIGLEY CORPORATION
CONSOLIDATED
BALANCE SHEETS
ASSETS
|
December
31, 2008
|
December
31, 2007
|
|||||||
|
CURRENT
ASSETS:
|
||||||||
|
Cash
and cash equivalents
|
$ | 11,956,796 | $ | 15,133,546 | ||||
|
Accounts
receivable (net of doubtful
accounts of $131,162 and $178,144)
|
4,523,519 | 6,648,538 | ||||||
|
Inventory
|
3,001,001 | 4,135,511 | ||||||
|
Prepaid
expenses and other current assets
|
1,185,113 | 810,106 | ||||||
|
Assets
of discontinued operations
|
- | 2,107,589 | ||||||
|
TOTAL CURRENT
ASSETS
|
20,666,429 | 28,835,290 | ||||||
|
PROPERTY, PLANT AND
EQUIPMENT –
net
|
3,666,748 | 4,337,540 | ||||||
|
OTHER
ASSETS:
|
||||||||
|
Other
assets
|
35,454 | 280,654 | ||||||
|
Assets
of discontinued operations
|
- | 48,437 | ||||||
|
TOTAL OTHER
ASSETS
|
35,454 | 329,091 | ||||||
|
TOTAL
ASSETS
|
$ | 24,368,631 | $ | 33,501,921 | ||||
LIABILITIES
AND STOCKHOLDERS’ EQUITY
|
CURRENT
LIABILITIES:
|
||||||||
|
Accounts
payable
|
$ | 693,839 | $ | 454,963 | ||||
|
Accrued
royalties and sales commissions
|
3,791,519 | 3,859,287 | ||||||
|
Accrued
advertising
|
1,306,341 | 1,369,759 | ||||||
|
Other
current liabilities
|
803,054 | 2,542,128 | ||||||
|
Liabilities
of discontinued operations
|
- | 2,031,529 | ||||||
|
TOTAL CURRENT
LIABILITIES
|
6,594,753 | 10,257,666 | ||||||
|
COMMITMENTS
AND CONTINGENCIES (Note 9)
|
||||||||
|
STOCKHOLDERS’
EQUITY:
|
||||||||
|
Common
stock, $.0005 par value;
authorized 50,000,000; Issued: 17,554,436
and 17,499,186 shares
|
8,777 | 8,750 | ||||||
|
Additional
paid-in-capital
|
37,599,405 | 37,535,523 | ||||||
|
Retained
earnings
|
5,353,855 | 10,888,141 | ||||||
|
Less:
Treasury stock, 4,646,053
and 4,646,053 shares, at cost
|
(25,188,159 | ) | (25,188,159 | ) | ||||
|
TOTAL STOCKHOLDERS’
EQUITY
|
17,773,878 | 23,244,255 | ||||||
|
TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$ | 24,368,631 | $ | 33,501,921 | ||||
See
accompanying notes to consolidated financial statements
THE
QUIGLEY CORPORATION
CONSOLIDATED
STATEMENTS OF OPERATIONS
|
Year
Ended
|
Year
Ended
|
Year
Ended
|
||||||||||
|
December
31, 2008
|
December
31, 2007
|
December
31, 2006
|
||||||||||
|
NET
SALES
|
$ | 20,506,612 | $ | 28,241,502 | $ | 26,850,030 | ||||||
|
COST
OF SALES
|
9,093,593 | 9,685,361 | 9,305,132 | |||||||||
|
GROSS
PROFIT
|
11,413,019 | 18,556,141 | 17,544,898 | |||||||||
|
OPERATING
EXPENSES:
|
||||||||||||
|
Sales
and marketing
|
5,958,031 | 4,994,947 | 6,812,630 | |||||||||
|
Administration
|
7,943,128 | 9,626,665 | 8,108,807 | |||||||||
|
Research
and development
|
4,241,724 | 6,482,485 | 3,787,498 | |||||||||
|
TOTAL
OPERATING EXPENSES
|
18,142,883 | 21,104,097 | 18,708,935 | |||||||||
|
LOSS
FROM OPERATIONS
|
(6,729,864 | ) | (2,547,956 | ) | (1,164,037 | ) | ||||||
|
OTHER
INCOME (EXPENSE)
|
||||||||||||
|
Interest
income
|
320,062 | 691,684 | 726,627 | |||||||||
|
Interest
expense
|
- | - | (21,644 | ) | ||||||||
|
TOTAL
OTHER INCOME, NET
|
320,062 | 691,684 | 704,983 | |||||||||
|
LOSS
FROM CONTINUING OPERATIONS BEFORE TAXES
|
(6,409,802 | ) | (1,856,272 | ) | (459,054 | ) | ||||||
|
INCOME
TAXES
|
- | - | 88,599 | |||||||||
|
LOSS
FROM CONTINUING OPERATIONS
|
(6,409,802 | ) | (1,856,272 | ) | (547,653 | ) | ||||||
|
DISCONTINUED
OPERATIONS:
|
||||||||||||
|
Gain
on disposal of health and wellness operations
|
736,252 | - | - | |||||||||
|
Income
(Loss) from discontinued operations
|
139,264 | (602,065 | ) | (1,200,692 | ) | |||||||
|
NET
LOSS
|
$ | (5,534,286 | ) | $ | (2,458,337 | ) | $ | (1,748,345 | ) | |||
|
(Loss)
Earnings per common share:
|
||||||||||||
|
Loss
from continuing operations
|
$ | (0.50 | ) | $ | (0.14 | ) | $ | (0.04 | ) | |||
|
Income
(Loss) from discontinued operations
|
$ | 0.07 | $ | (0.05 | ) | $ | (0.10 | ) | ||||
|
Net
Loss
|
$ | (0.43 | ) | $ | (0.19 | ) | $ | (0.14 | ) | |||
|
Diluted
earnings per common share:
|
||||||||||||
|
Loss
from continuing operations
|
$ | (0.50 | ) | $ | (0.14 | ) | $ | (0.04 | ) | |||
|
Income
(Loss) from discontinued operations
|
$ | 0.07 | $ | (0.05 | ) | $ | (0.10 | ) | ||||
|
Net
Loss
|
$ | (0.43 | ) | $ | (0.19 | ) | $ | (0.14 | ) | |||
|
Weighted
average common shares outstanding:
|
||||||||||||
|
Basic
|
12,877,983 | 12,728,706 | 12,245,073 | |||||||||
|
Diluted
|
12,877,983 | 12,728,706 | 12,245,073 | |||||||||
See
accompanying notes to consolidated financial statements
THE
QUIGLEY CORPORATION
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ EQUITY
|
Common
Stock
Shares
|
Issued
Amount
|
Additional
Paid-in-
Capital
|
Treasury
Stock
|
Retained
Earnings
|
Total
|
||||||||||||||||||||
|
Balance
December 31, 2005
|
11,714,471 | $ | 8,180 | $ | 35,404,803 | $ | (25,188,159 | ) | $ | 15,094,823 | $ | 25,319,647 | |||||||||||||
|
Tax
benefits from options, warrants
& common stock
|
2,484,330 | 2,484,330 | |||||||||||||||||||||||
|
Tax
benefit allowance
|
(2,484,330 | ) | (2,484,330 | ) | |||||||||||||||||||||
|
Proceeds
from options and warrants
exercised
|
1,011,155 | 505 | 1,957,630 | 1,958,135 | |||||||||||||||||||||
|
Stock
Cancellation
|
(40,993 | ) | (20 | ) | 20 | - | |||||||||||||||||||
|
Net
Loss
|
(1,748,345 | ) | (1,748,345 | ) | |||||||||||||||||||||
|
Balance
December 31, 2006
|
12,684,633 | 8,665 | 37,362,453 | (25,188,159 | ) | 13,346,478 | 25,529,437 | ||||||||||||||||||
|
Tax
benefits from options, warrants
& common stock
|
153,631 | 153,631 | ||||||||||||||||||||||
|
Tax
benefit allowance
|
(153,631 | ) | (153,631 | ) | ||||||||||||||||||||
|
Proceeds
from options and warrants
exercised
|
168,500 | 85 | 173,070 | 173,155 | ||||||||||||||||||||
|
Net
Loss
|
(2,458,337 | ) | (2,458,337 | ) | ||||||||||||||||||||
|
Balance
December 31, 2007
|
12,853,133 | 8,750 | 37,535,523 | (25,188,159 | ) | 10,888,141 | 23,244,255 | |||||||||||||||||
|
Tax
benefits from options, warrants
& common stock
|
67,717 | 67,717 | ||||||||||||||||||||||
|
Tax
benefit allowance
|
(67,717 | ) | (67,717 | ) | ||||||||||||||||||||
|
Proceeds
from options exercised
|
55,250 | 27 | 63,882 | 63,909 | ||||||||||||||||||||
|
Net
Loss
|
(5,534,286 | ) | (5,534,286 | ) | ||||||||||||||||||||
|
Balance
December 31, 2008
|
12,908,383 | $ | 8,777 | $ | 37,599,405 | $ | (25,188,159 | ) | $ | 5,353,855 | $ | 17,773,878 | ||||||||||||
See
accompanying notes to consolidated financial statements
THE
QUIGLEY CORPORATION
CONSOLIDATED
STATEMENTS OF CASH FLOWS
|
Year
Ended
December
31, 2008
|
Year
Ended
December
31, 2007
|
Year
Ended
December
31, 2006
|
||||||||||
|
OPERATING
ACTIVITIES:
|
||||||||||||
|
Net
loss
|
$ | (5,534,286 | ) | $ | (2,458,337 | ) | $ | (1,748,345 | ) | |||
|
Adjustments
to reconcile net loss to net cash provided
by continuing operations:
|
||||||||||||
|
Loss
on asset impairment
|
100,000 | - | - | |||||||||
|
Depreciation
and amortization
|
743,670 | 937,852 | 1,145,792 | |||||||||
|
Loss
on the sales of fixed assets
|
10,188 | - | - | |||||||||
|
Bad
debts provision
|
(403 | ) | 8,647 | (14,901 | ) | |||||||
|
(Increase)
decrease in assets:
|
||||||||||||
|
Accounts
receivable
|
2,125,436 | (139,741 | ) | 1,282,751 | ||||||||
|
Inventory
|
1,134,510 | (781,098 | ) | (88,188 | ) | |||||||
|
Prepaid
expenses and other current assets
|
(375,007 | ) | 7,504 | 333,268 | ||||||||
|
Other
assets
|
245,200 | (97,766 | ) | (72,031 | ) | |||||||
|
Increase
(decrease) in liabilities:
|
||||||||||||
|
Accounts
payable
|
238,876 | (206,992 | ) | 120,415 | ||||||||
|
Accrued
royalties and sales commissions
|
(67,768 | ) | 342,788 | 494,548 | ||||||||
|
Accrued
advertising
|
(63,418 | ) | (770,498 | ) | (710,155 | ) | ||||||
|
Other
current liabilities
|
(1,739,074 | ) | 1,288,253 | (232,906 | ) | |||||||
|
Total
adjustments
|
2,352,210 | 588,949 | 2,258,593 | |||||||||
|
NET
CASH (USED) PROVIDED BY OPERATING ACTIVITIES
|
(3,182,076 | ) | (1,869,388 | ) | 510,248 | |||||||
|
INVESTING
ACTIVITIES:
|
||||||||||||
|
Capital
expenditures
|
(199,764 | ) | (521,287 | ) | (587,642 | ) | ||||||
|
Proceeds
from the sale of fixed assets
|
16,697 | - | 118,276 | |||||||||
|
NET
CASH FLOWS USED IN INVESTING ACTIVITIES
|
(183,067 | ) | (521,287 | ) | (469,366 | ) | ||||||
|
FINANCING
ACTIVITIES:
|
||||||||||||
|
Principal
payments on debt
|
- | - | (1,464,286 | ) | ||||||||
|
Stock
options and warrants exercised
|
63,909 | 173,155 | 1,958,135 | |||||||||
|
NET
CASH FLOWS PROVIDED BY FINANCING
ACTIVITIES
|
63,909 | 173,155 | 493,849 | |||||||||
|
DISCONTINUED
OPERATIONS:
|
||||||||||||
|
(Gain)
Loss from discontinued operations
|
(875,516 | ) | 1,060,447 | (628,000 | ) | |||||||
| Proceeds from sale of discontinued operations | 1,000,000 | - | - | |||||||||
|
NET
CASH FLOWS PROVIDED (USED) BY DISCONTINUED
OPERATIONS
|
124,484 | 1,060,447 | (628,000 | ) | ||||||||
|
NET
DECREASE IN CASH & CASH EQUIVALENTS
|
(3,176,750 | ) | (1,157,073 | ) | (93,269 | ) | ||||||
|
CASH
& CASH EQUIVALENTS, BEGINNING OF PERIOD
|
15,133,546 | 16,290,619 | 16,383,888 | |||||||||
|
CASH
& CASH EQUIVALENTS, END
OF PERIOD
|
$ | 11,956,796 | $ | 15,133,546 | $ | 16,290,619 | ||||||
|
SUPPLEMENTAL
DISCLOSURE OF CASH FLOW INFORMATION:
|
||||||||||||
|
Cash
paid for:
|
||||||||||||
|
Interest
|
$ | - | $ | - | $ | 21,644 | ||||||
|
Taxes
|
$ | - | $ | - | $ | 88,599 | ||||||
See
accompanying notes to consolidated financial statements
THE
QUIGLEY CORPORATION
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND
BUSINESS
The
Company, headquartered in Doylestown, Pennsylvania, is a leading manufacturer,
marketer and distributor of a diversified range of homeopathic and health
products which comprise the Cold Remedy and Contract Manufacturing
segments. The Company is also involved in the research and
development of potential prescription and other medicinal products that comprise
the Ethical Pharmaceutical segment.
The
Company’s business is the manufacture and distribution of cold remedy products
to the consumer through the over-the-counter marketplace. One of the Company’s
key products in its Cold Remedy segment is Cold-EezeÒ, a zinc gluconate glycine
product proven in two double-blind clinical studies to reduce the duration and
severity of the common cold symptoms by nearly half. Cold-Eeze® is now an
established product in the health care and cold remedy market.
Effective
October 1, 2004, the Company acquired substantially all of the assets of JoEl,
Inc., the previous manufacturer of the Cold-EezeÒ lozenge
product. This manufacturing entity, now called Quigley Manufacturing
Inc. (“QMI”), a wholly-owned subsidiary of the Company, will continue to produce
lozenge product along with performing such operational tasks as warehousing and
shipping the Company’s Cold-EezeÒ products. In
addition, QMI produces a variety of hard and organic candy for sale to third
party customers in addition to performing contract manufacturing activities for
non-related entities. On February 2, 2009, the Company announced its
intention to close the Elizabethtown location of Quigley Manufacturing Inc., and
discontinue the hard candy business resulting in the consolidation of
manufacturing operations at the Lebanon location. This consolidation
will have no impact on the production or distribution of the Cold-EezeÒ brand of cold remedy
products.
In
January 2001, the Company formed an Ethical Pharmaceutical segment, Quigley
Pharma Inc. (“Pharma”), that is under the direction of its Executive Vice
President and Chairman of its Medical Advisory Committee. Pharma was
formed for the purpose of research and development of potential natural base
health products, including, but not limited to, prescription medicines along
with supplements and cosmeceuticals for human and veterinary
use. Pharma is currently undergoing research and development activity
in compliance with regulatory requirements. The Company is in the initial stages
of what may be a lengthy process to develop these patent applications into
commercial products.
On
February 29, 2008, the Company sold Darius International Inc. (“Darius”) to
InnerLight Holdings, Inc., whose major shareholder is Mr. Kevin P. Brogan, the
then president of Darius. Darius marketed health and wellness
products through its wholly-owned subsidiary, Innerlight Inc. that constituted
the Health and Wellness segment of the Company. The terms of the sale
agreement included a cash purchase price of $1,000,000 by InnerLight Holdings,
Inc. for the stock of Darius and its subsidiaries without guarantees, warranties
or indemnifications. Financial information related to this former
segment is presented as Discontinued Operations. See discussion in
Note 3 to Consolidated Financial Statements.
Future
revenues, costs, margins, and profits will continue to be influenced by the
Company’s ability to maintain its manufacturing availability and capacity
together with its marketing and distribution capabilities and the requirements
associated with the development of Pharma’s potential prescription drugs and
other medicinal products in order to continue to compete on a national and
international level. The business development of the Company is
dependent on continued conformity with government regulations, a reliable
information technology system capable of supporting continued growth and
continued reliable sources for product and materials to satisfy consumer
demand.
The
business of the Company is subject to federal and state laws and regulations
adopted for the health and safety of users of the Company’s products. Cold-Eeze®
is a homeopathic remedy that is subject to regulations by various federal, state
and local agencies, including the FDA and the Homeopathic Pharmacopoeia of the
United States.
NOTE 2 – SUMMARY OF SIGNIFICANT
ACCOUNTING POLICIES
Basis
of Presentation
The
Consolidated Financial Statements include the accounts of the Company and its
wholly-owned subsidiaries. All inter-company transactions and balances have been
eliminated. Effective March 31, 2004, the financial statements
include consolidated variable interest entities (“VIEs”) of which the Company is
the primary beneficiary. (See discussion in Note 4, “Variable
Interest Entity.”)
Use
of Estimates
The
Company’s consolidated financial statements are prepared in accordance with
generally accepted accounting principles (GAAP) in the United Sates of America.
In connection with the preparation of the consolidated financial statements, the
Company is required to make assumptions and estimates about future events, and
apply judgments that affect the reported amounts of assets, liabilities,
revenue, expenses and related disclosures. These assumptions, estimates and
judgments are based on historical experience, current trends and other factors
that management believes to be relevant at the time the consolidated financial
statements are prepared. Management reviews the accounting policies,
assumptions, estimates and judgments on a quarterly basis to ensure the
financial statements are presented fairly and in accordance with GAAP. However,
because future events and their effects cannot be determined with certainty,
actual results could differ from these assumptions and estimates, and such
differences could be material.
The
Company is organized into three different but related business segments, Cold
Remedy, Contract Manufacturing and Ethical Pharmaceutical. When providing for
the appropriate sales returns, allowances, cash discounts and cooperative
incentive program costs, each segment applies a uniform and consistent method
for making certain assumptions for estimating these provisions that are
applicable to each specific segment. Traditionally, these provisions are not
material to reported revenues in the Contract Manufacturing segments and the
Ethical Pharmaceutical segment does not have any revenues.
Provisions
to these reserves within the Cold Remedy segment include the use of such
estimates, which are applied or matched to the current sales for the period
presented. These estimates are based on specific customer tracking and an
overall historical experience to obtain an applicable effective rate. Estimates
for sales returns are tracked at the specific customer level and are tested on
an annual historical basis, and reviewed quarterly, as is the estimate for
cooperative incentive promotion costs. Cash discounts follow the terms of sales
and are taken by virtually all customers. Additionally, the monitoring of
current occurrences, developments by customer, market conditions and any other
occurrences that could affect the expected provisions for any future returns or
allowances, cash discounts and cooperative incentive promotion costs relative to
net sales for the period presented are also performed.
Cash
Equivalents
The
Company considers all highly liquid investments with an initial maturity of
three months or less at the time of purchase to be cash
equivalents. Cash equivalents include cash on hand and monies
invested in money market funds. The carrying amount approximates the fair market
value due to the short-term maturity of these investments.
Inventories
Inventory
is valued at the lower of cost, determined on a first-in, first-out basis
(FIFO), or market. Inventory items are analyzed to determine cost and the market
value and appropriate valuation reserves are established. The consolidated
financial statements include a reserve for excess or obsolete inventory of
$1,200,803 and $368,491 as of December 31, 2008 and 2007,
respectively. Inventories included raw material, work in progress and
packaging amounts of approximately $975,000 and $1,197,000 at December 31, 2008
and December 31, 2007, respectively, with the remainder comprising finished
goods.
Property,
Plant and Equipment
Property,
plant and equipment are recorded at cost. The Company uses a
combination of straight-line and accelerated methods in computing depreciation
for financial reporting purposes. The annual provision for
depreciation has been computed in accordance with the following ranges of
estimated asset lives: building and improvements - twenty to thirty nine years;
machinery and equipment - five to seven years; computer software - three years;
and furniture and fixtures – seven years.
Concentration
of Risks
Financial
instruments that potentially subject the Company to significant concentrations
of credit risk consist principally of cash investments and trade accounts
receivable.
The
Company maintains cash and cash equivalents with several major financial
institutions. Due to the nature of the funds maintained by the Company, all fund
balances are completely guaranteed due to the Temporary Guarantee Program for
Money Market Funds and the unlimited FDIC coverage available to non-interest
bearing transaction accounts. The Company will continue to monitor
these programs as they contain future expiry dates and to limit the amount of
credit exposure with any one financial institution.
Trade
accounts receivable potentially subjects the Company to credit
risk. The Company extends credit to its customers based upon an
evaluation of the customer’s financial condition and credit history and
generally does not require collateral. The Company’s broad range of customers
includes many large wholesalers, mass merchandisers and multi-outlet pharmacy
chains, five of which account for a significant percentage of sales volume,
representing 48% for the year ended December 31, 2008, 49% for the year ended
December 31, 2007, and 47% for the year ended December 31,
2006. Customers comprising the five largest accounts receivable
balances represented 55% and 40% of total trade receivable balances at December
31, 2008 and 2007, respectively. During 2008, 2007 and 2006,
effectively all of the Company’s revenues were related to domestic
markets.
The
Company’s revenues are currently generated from the sale of the Cold Remedy
products which approximated 89%, 91% and 92% of total revenues in the twelve
month periods ended December 31, 2008, 2007 and 2006, respectively. The Contract
Manufacturing segment approximated 11%, 9% and 8% for the year ended December
31, 2008, 2007 and 2006, respectively.
Raw
materials used in the production of the products are available from numerous
sources. Raw materials for the Cold-EezeÒ lozenge product are
currently procured from a single vendor in order to secure purchasing
economies. In a situation where this one vendor is not able to supply
QMI with the ingredients, other sources have been identified. Should
these product sources terminate or discontinue for any reason, the Company has
formulated a contingency plan in order to prevent such discontinuance from
materially affecting the Company’s operations. Any such termination
may, however, result in a temporary delay in production until the replacement
facility is able to meet the Company’s production requirements.
Long-lived
Assets
The
Company reviews its long-lived assets for impairment on an exception basis
whenever events or changes in circumstances indicate that the carrying amount of
the assets may not be recoverable through future undiscounted cash
flows. In 2008, the Company recognized an impairment charge of
$300,000 due to adverse profit margins related to the hard candy business of QMI
with such expense reflected in cost of sales.
Revenue
Recognition
Sales are
recognized at the time ownership is transferred to the customer, which for the
Cold Remedy segment is the time the shipment is received by the customer and for
the Contract Manufacturing segment, when the product is shipped to the
customer. Revenue is reduced for trade promotions, estimated sales
returns, cash discounts and other allowances in the same period as the related
sales are recorded. The Company makes estimates of potential future product
returns and other allowances related to current period revenue. The Company
analyzes historical returns, current trends, and changes in customer and
consumer demand when evaluating the adequacy of the sales returns and other
allowances. The consolidated financial statements include reserves of $1,427,045
for future sales returns and $280,973 for other allowances as of December 31,
2008 and $295,606 and $347,103 at December 31, 2007, respectively. The
reserves also include an estimate of the uncollectability of accounts receivable
resulting in a reserve of $131,162 at December 31, 2008 and $178,144 at December
31, 2007.
Cost
of Sales
For the
Cold Remedy segment, in accordance with contract terms, payments calculated
based upon net sales collected to the patent holder of the Cold-Eeze formulation
and payments to the corporation founders (this agreement terminated in 2005) and
developers of the final saleable Cold-Eeze product (this agreement terminated in
2007) amounting to zero, $293,266 and $1,153,354, respectively, at December 31,
2008, 2007 and 2006 are presented in the financial statements as cost of
sales.
Operating
expenses
Agreements
relating to the Cold Remedy segment with a major national sales brokerage firm
are for this firm to sell the manufactured Cold-Eeze product to our customers.
Such related costs are presented in the financial statements as selling
expenses.
Shipping
and Handling
Product
sales relating to the Cold Remedy and Contract Manufacturing segments carry
shipping and handling charges to the purchaser, included as part of the invoiced
price, which is classified as revenue. In all cases costs related to
this revenue are recorded in cost of sales.
Stock
Compensation
Stock
options and warrants for purchase of the Company’s common stock have been
granted to both employees and non-employees since the date the Company became
publicly traded. Options and warrants are exercisable during a period
determined by the Company, but in no event later than ten years from the date
granted.
No stock
options were granted to employees and non-employees in 2008, 2007 and 2006,
respectively.
Advertising
and Incentive Promotions
Advertising
and incentive promotion costs are expensed within the period in which they are
utilized. Advertising and incentive promotion expense is comprised of media
advertising, presented as part of sales and marketing expense; co-operative
incentive promotions and coupon program expenses, which are accounted for as
part of net sales; and free product, which is accounted for as part of cost of
sales. Advertising and incentive promotion costs incurred for the
years ended December 31, 2008, 2007 and 2006 were $7,654,452, $7,290,065, and
$7,703,426, respectively. Included in prepaid expenses and other
current assets was $241,971 and $158,428 at December 31, 2008 and 2007 relating
to prepaid advertising and promotion expenses.
Research
and Development
Research
and development costs are charged to operations in the period incurred.
Expenditures for the years ended December 31, 2008, 2007 and 2006 were
$4,241,724, $6,482,485 and $3,787,498, respectively. Principally,
research and development costs are related to Pharma’s study activities and
costs associated with Cold-EezeÒ.
Income
Taxes
The
Company utilizes the asset and liability approach which requires the recognition
of deferred tax assets and liabilities for the future tax consequences of events
that have been recognized in the Company’s financial statements or tax returns.
In estimating future tax consequences, the Company generally considers all
expected future events other than enactments of changes in the tax law or
rates. Until sufficient taxable income to offset the temporary timing
differences attributable to operations and the tax deductions attributable to
option, warrant and stock activities are assured, a valuation allowance equaling
the total deferred tax asset is being provided. (See Note 13 –
“Income Taxes” for further discussion.)
Effective
January 1, 2007, the Company adopted Financial Interpretation ("FIN") No. 48,
Accounting for Uncertainty in
Income Taxes - An Interpretation of FASB Statement No. 109. This
interpretation prescribes a recognition threshold and measurement attribute for
the financial statement recognition and measurement of a tax position taken or
expected to be taken in a tax return. The interpretation contains a two-step
approach to recognizing and measuring uncertain tax positions accounted for in
accordance with SFAS No. 109. The first step is to evaluate the tax
position for recognition by determining if the weight of available evidence
indicates that it is more likely than not that the position will be sustained on
audit, including resolution of related appeals or litigation processes, if any.
The second step is to measure the tax benefit as the largest amount which is
more than fifty percent likely of being realized upon ultimate settlement. The
interpretation also provides guidance on derecognition, classification, interest
and penalties, and other matters. The adoption did not have an effect on the
consolidated financial statements.
As a
result of the Company’s continuing tax losses, the Company has recorded a full
valuation allowance against a net deferred tax asset. Additionally,
the Company has not recorded a liability for unrecognized tax benefits for
December 31, 2008 and 2007.
The major
jurisdiction for which the Company files income tax returns is the United
States. The Internal Revenue Service has examined the Company’s tax
year ended September 30, 2005 and has made no changes to the filed tax
returns. The tax years 2004 and forward remain open to examination by
the various taxing authorities to which the Company is subject.
Fair
Value of Financial Instruments
Cash and
cash equivalents, accounts receivable and accounts payable are reflected in the
consolidated financial statements at carrying value which approximates fair
value because of the short-term maturity of these instruments. The
fair value of past periods’ long-term debt was approximately equivalent to its
carrying value due to the fact that the interest rates then available to the
Company for debt with similar terms were approximately equal to the interest
rates for the Company’s debt. Determination of the fair value of
related party payables is not practicable due to their related party
nature.
Recently
Issued Accounting Standards
In
September 2006, the Financial Accounting Standards Board (FASB) issued
Statement of Financial Accounting Standards No. 157, “Fair Value Measurements”
(“SFAS 157”). SFAS 157 defines fair value, establishes a framework for
measuring fair value in generally accepted accounting principles (GAAP) and
expands disclosures about fair value measurements. SFAS 157 is effective
for fiscal years beginning after November 15, 2007 and interim periods
within those fiscal years. The adoption of this standard has not had a
significant impact on the Company’s consolidated financial position, results of
operations or cash flows.
In
February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial
Assets and Financial Liabilities”, including an amendment of FASB No. 115
("FAS 159"). The Statement permits companies to choose to measure many financial
instruments and certain other items at fair value in order to mitigate
volatility in reported earnings caused by measuring related assets and
liabilities differently without having to apply complex hedge accounting
provisions. FAS 159 is effective for the Company beginning January 1, 2008. The
adoption of this standard has not had a significant impact on the Company’s
consolidated financial position, results of operations or cash
flows.
In
December 2007, the FASB issued Statement of Financial Accounting Standard
No. 160, “Noncontrolling
Interests in Consolidated Financial Statements — an amendment of ARB
No. 51” (“FAS 160”). FAS 160 establishes accounting and
reporting standards for the non-controlling interest in a subsidiary and for the
retained interest and gain or loss when a subsidiary is deconsolidated. This
statement is effective for financial statements issued for fiscal years
beginning on or after December 15, 2008 with earlier adoption prohibited.
The adoption of this standard is not expected to have a significant impact on
the Company’s consolidated financial position, results of operations or cash
flows.
In
December 2007, the FASB issued SFAS No. 141R, "Business Combinations,"
(“SFAS 141R”) which establishes principles and requirements for how an
acquirer recognizes and measures in its financial statements the identifiable
assets acquired, the liabilities assumed and any non-controlling interest in the
acquiree. SFAS 141R also establishes disclosure requirements to enable the
evaluation of the nature and financial effects of the business combination. SFAS
141R applies prospectively to business combinations for which the acquisition
date is on or after the beginning of the first annual reporting period beginning
on or after December 15, 2008, and interim periods
within those fiscal years.
The adoption of this standard will not have any impact on the Company’s
consolidated financial position, results of operations or cash
flows.
NOTE
3 – DISCONTINUED OPERATIONS
On
February 29, 2008, the Company sold Darius to InnerLight Holdings, Inc., whose
major shareholder is Mr. Kevin P. Brogan, the then president of
Darius. The Quigley Corporation formed Darius in 2000 to introduce
new products to the marketplace through a network of independent distributor
representatives. Darius marketed health and wellness products through
its wholly-owned subsidiary, Innerlight Inc. that constituted the Health and
Wellness segment of the Company. The terms of the sale agreement
include a cash purchase price of $1,000,000 by InnerLight Holdings, Inc. for the
stock of Darius and its subsidiaries without guarantees, warranties or
indemnifications.
Sales of
Darius in 2008 until date of disposal on February 29, 2008 and for the twelve
month periods ended December 31, 2007 and 2006 were, respectively, $2,188,815,
$11,233,879 and $15,274,940. Net income (losses) for 2008 until date
of disposal on February 29, 2008 and for the twelve month periods ended December
31, 2007 and 2006, were $139,264, ($602,065) and ($1,200,692),
respectively. Results of Darius are presented as discontinued
operations in the Consolidated Statements of Operations and Cash Flows and in
the Consolidated Balance Sheets. The major classes of balance sheet items of
discontinued operations at December 31, 2007 were cash, inventory, prepaid
expenses and other current liabilities.
The
Company recorded a gain on the disposal of Darius of $736,252, as a result of
sales proceeds of $1,000,000 less residual investment of $5,000 and net assets
of Darius of $258,748 on the date of sale.
NOTE
4 – VARIABLE INTEREST ENTITY
In
December 2003, the Financial Accounting Standards Board (FASB or the “Board”)
issued FASB Interpretation No. 46 (revised December 2003), Consolidation of Variable Interest
Entities (FIN 46R), to address certain implementation
issues. FIN 46R varies significantly from FASB Interpretation No. 46,
Consolidation of Variable
Interest Entities (“VIE”) (FIN 46), which it supersedes. FIN
46R requires the application of either FIN 46 or FIN 46R by “Public
Entities” to
all Special Purpose Entities (“SPEs”)
at the end of the first interim or annual
reporting period ending after December 15, 2003. FIN 46R
is applicable to all non-SPEs created prior to
February 1, 2003 by Public Entities that are not small
business issuers at the end of the first interim or annual reporting period
ending after March 15, 2004. Effective March 31, 2004, the Company
adopted FIN 46R for VIE’s formed prior to February 1, 2003. The
Company had determined that Scandasystems, a related party, qualified as a
variable interest entity and the Company consolidated Scandasystems beginning
with the quarter ended March 31, 2004. Due to the fact that the
Company had no long-term contractual commitments or guarantees, the maximum
exposure to loss was insignificant.
The
Company has determined that the conditions that applied in the past giving rise
to the application of FIN 46R to the relationship between the Company and
Scandasystems no longer apply. Therefore, effective with quarter
ended March 31, 2008, Scandasystems balances were no longer consolidated with
the Company’s financial results and balances.
NOTE
5 – PROPERTY, PLANT AND EQUIPMENT
Consisted of the following as
of:
|
December
31, 2008
|
December
31, 2007
|
|||||||
|
Land
|
$ | 538,791 | $ | 538,791 | ||||
|
Buildings
and improvements
|
2,691,610 | 2,688,158 | ||||||
|
Machinery
and equipment
|
4,933,197 | 4,988,292 | ||||||
|
Computer
software
|
134,007 | 113,013 | ||||||
|
Furniture
and fixtures
|
238,788 | 235,544 | ||||||
| 8,536,393 | 8,563,798 | |||||||
|
Less:
Accumulated depreciation
|
4,869,645 | 4,226,258 | ||||||
|
Property,
Plant and Equipment, net
|
$ | 3,666,748 | $ | 4,337,540 | ||||
Depreciation
expense for the years ended December 31, 2008, 2007 and 2006 was $743,670,
$937,852, and $1,145,792, respectively. During the year ended
December 31, 2008, the Company retired equipment with an original cost of
approximately $127,169 and accumulated depreciation of approximately
$100,283. In addition, an amount of $100,000 was recorded during the
year ended December 31, 2008 representing impairment costs of fixed assets at
the Elizabethtown, Pennsylvania, manufacturing facility.
NOTE
6 – PATENT RIGHTS AND RELATED ROYALTY COMMITMENTS
The
Company has maintained a separate representation and distribution agreement
relating to the development of the zinc gluconate glycine product
formulation. In return for exclusive distribution rights, the Company
must pay the developer a 3% royalty and a 2% consulting fee based on sales
collected, less certain deductions, throughout the term of this agreement, which
expired May 2007. However, the Company and the developer are in
litigation (see Note 9) and as such no potential offset for these fees from such
litigation has been recorded.
The
expense for the respective periods relating to this agreement amounted to zero,
$293,266 and $1,153,354, for the years ended December 31, 2008, 2007 and 2006,
respectively. Amount accrued for this expense at December 31, 2008
and 2007 was $3,524,031, on both dates.
NOTE
7 – LONG-TERM DEBT
In
connection with the Company’s acquisition of certain assets of JoEl, Inc. in
October 2004, the Company entered into a term loan in the amount of $3 million
payable to PNC Bank, N.A. which was collateralized by mortgages on real property
located in each of Lebanon and Elizabethtown, Pennsylvania. The
Company could elect interest rate options at either the Prime Rate or LIBOR plus
200 basis points. The loan was payable in eighty-four equal monthly
principal payments of $35,714 that commenced on November 1, 2004. In
April 2005, the Company prepaid an amount of $1.0 million against the
outstanding balance on the long-term loan. In April 2006, the Company
prepaid the total outstanding balance of approximately $1.3
million.
NOTE 8 – OTHER CURRENT
LIABILITIES
Included
in other current liabilities are $215,350 and $1,240,767 related to accrued
compensation at December 31, 2008 and 2007, respectively.
NOTE 9 – COMMITMENTS AND
CONTINGENCIES
Certain
operating leases for office and warehouse space maintained by the Company
resulted in rent expense for the years ended December 31,
2008, 2007 and 2006,
of $53,200, $68,436, and $60,735, respectively. The
Company has approximate
future obligations over the next five years as follows:
|
Year
|
Research
and Development
|
Property
and Other Leases
|
Advertising
|
Product
Purchases
|
Total
|
|||||||||||||||
|
2009
|
$ | 442,000 | $ | 19,406 | $ | 1,920,173 | $ | 1,355,000 | $ | 3,736,579 | ||||||||||
|
2010
|
- | - | - | 1,321,000 | 1,321,000 | |||||||||||||||
|
2011
|
- | - | - | 671,000 | 671,000 | |||||||||||||||
|
2012
|
- | - | - | - | - | |||||||||||||||
|
2013
|
- | - | - | - | - | |||||||||||||||
|
Total
|
$ | 442,000 | $ | 19,406 | $ | 1,920,173 | $ | 3,347,000 | $ | 5,728,579 | ||||||||||
Additional
advertising and research and development costs are expected to be incurred
during the remainder of 2009.
During
July 2008, the Company entered into an agreement with a vendor to purchase a
minimum order of product, with the amount of approximately $3,347,000 remaining,
over a three year period in its capacity as an exclusive reseller, marketer and
distributor of a cough and cold product incorporating a patented, proprietary
delivery system.
On July
2, 2008, the Company entered into an agreement with Dr. Richard Rosenbloom,
Executive Vice President and Chief Operating Officer of Pharma, whereby the
Company agreed to compensate Dr. Rosenbloom for assigning, to the Company, the
entire right, title and interest in and to Dr. Rosenbloom’s concepts and/or
inventions made prior to the date he became an employee of The Quigley
Corporation. In consideration of, and as full compensation for, the
covenants made in the agreement, the Company shall pay Dr. Rosenbloom
compensation in the amount of five percent (5%) of net sales collected, less
certain deductions, of royalty bearing products. This agreement has
no current financial impact to the Company due to the absence of Pharma related
sales.
The
Company has had other contractual agreements. (See Note
6.)
TESAURO
AND ELEY, ET AL. VS. THE QUIGLEY CORPORATION
(CCP
of Phila., August Term 2000, No. 001011)
In
September 2000, the Company was sued by two individuals (Jason Tesauro and
Elizabeth Eley, both residents of Georgia), allegedly on behalf of a "nationwide
class" of "similarly situated individuals," in the Court of Common Pleas of
Philadelphia County, Pennsylvania. The Complaint further alleges that
the plaintiffs purchased certain Cold-Eeze products between August 1996, and
November 1999, based upon cable television, radio and internet advertisements,
which allegedly misrepresented the qualities and benefits of the Company's
products. The Complaint, as pleaded originally, requested an
unspecified amount of damages for violations of Pennsylvania's consumer
protection law, breach of implied warranty of merchantability and unjust
enrichment, as well as a judicial determination that the action be maintained as
a class action. In October 2000, the Company filed Preliminary
Objections to the Complaint seeking dismissal of the action. The
court sustained certain objections, thereby narrowing plaintiffs'
claims.
In May
2001, plaintiffs filed a motion to certify the putative class. The
Company opposed the motion. In November 2001, the court held a
hearing on plaintiffs' motion for class certification. In January
2002, the court denied in part and granted in part plaintiffs'
motion. The court denied plaintiffs' motion to certify a class based
on plaintiffs' claims under Pennsylvania's consumer protection law, under which
plaintiffs sought treble damages, effectively dismissing this cause of action;
however, the court certified a class based on plaintiffs' secondary breach of
implied warranty and unjust enrichment claims. In August, 2002, the
court issued an order adopting a form of Notice of Class Action to be published
nationally. Significantly, the form of Notice approved by the court
included a provision which limits the potential class members who may
potentially recover damages in this action to those persons who present a proof
of purchase of Cold-Eeze during the period August 1996 and November
1999.
Afterward,
a series of pre-trial motions were filed raising issues concerning trial
evidence and the court's jurisdiction over the subject matter of the
action. In March, 2005, the court held oral argument on these
motions.
Significantly,
on November 8, 2006, the Court entered an Order dismissing the case in its
entirety on the basis that the action was preempted by federal
law. The plaintiffs appealed the Court's decision in December, 2006
to the Superior Court of the Commonwealth of
Pennsylvania. On February 19, 2008, the Superior Court upheld
defendant's appeal and remanded
the case to the Philadelphia County Court of Common Pleas for
trial.
The case
commenced trial on February 2, 2009. On February 6, 2009, the jury
returned a verdict in favor of the Company on all counts. Plaintiffs
had to February 17, 2009, to file post-trial motions, the first step in the
appeal process. No post-trial motions were filed by the
plaintiffs. At this time the Company has no notice as to whether the
plaintiffs will attempt to perfect an appeal.
THE
QUIGLEY CORPORATION VS. JOHN C. GODFREY, ET AL.
(Bucks
Co. CCP, No. 04-07776)
In this
action, which was commenced in November 2004, the Company is seeking declaratory
and injunctive relief against John C. Godfrey, Nancy Jane Godfrey, and Godfrey
Science and Design, Inc. requesting injunctive relief regarding the Cold-Eeze
trade name and trademark; injunctive relief relating to the Cold-Eeze
formulations and manufacturing methods; injunctive relief for breach of the duty
of loyalty, and declaratory judgment pending the Company's payment of
commissions to defendants. The Company's Complaint is based in part
upon the Exclusive Representation and Distribution Agreement and the Consulting
Agreement (together the "Agreements") entered into between the defendants and
the Company. The Company terminated the Agreements for the
defendants' alleged material breaches of the Agreements. Defendants
have answered the complaint and asserted counterclaims against the Company
seeking remedies relative to the Agreements. The Company believes
that the defendants' counterclaims are without merit and is vigorously defending
those counterclaims and is prosecuting its action on its complaint.
The
discovery phase of pre-trial discovery is nearing
completion. Defendants moved for partial summary judgment, and the
Company filed a response and cross-motion for summary judgment. On
August 21, 2008, the court denied both motions for summary
judgment. The case has not been assigned to a trial calendar,
although it is possible that the case will be listed for trial in
2009.
At this time no prediction as to the
outcome of this action can be made.
NICODROPS,
INC. VS. QUIGLEY MANUFACTURING, INC.
On
January 30, 2006, Quigley Manufacturing, Inc., a wholly-owned subsidiary of The
Quigley Corporation, was put on notice of a claim by Nicodrops,
Inc. Nicodrops, Inc. has claimed that the packaging contained
incorrect expiration dates and caused it to lose sales through two (2)
retailers. The total alleged sales of Nicodrops was approximately
$250,000 and Nicodrops is claiming unspecified damages exceeding
$2,000,000.
No suit
has been filed. The Company is investigating this
claim. Based on its investigation to date, the Company believes the
claim is without merit. However, at this time no prediction can be
made as to the outcome of this case.
THE
QUIGLEY CORPORATION VS. WACHOVIA INSURANCE SERVICES, INC. AND FIRST
UNION
INSURANCE SERVICES AGENCY, INC.
The
Quigley Corporation instituted a Writ of Summons against Wachovia Insurance
Services, Inc. and First Union Insurance Services Agency, Inc. on December 8,
2005. The purpose of this suit was to maintain an action and toll the
statute of limitation against The Quigley Corporation's insurance broker who
failed to place excess limits coverage for the Company for the period from
November 29, 2003 until April 6, 2004. As a result of the defendant's
failure to place insurance and to notify the Company of its actions, certain
pending actions covered by the Company's underlying
insurance at the present time may result in certain cases presently being
defended by insurance counsel and the underlying insurance carrier to cause an
exhaustion of the underlying insurance for the policy periods ending November
29, 2004 and November 29, 2005. Any case in which an alleged action
arose by the use of COLD-EEZE Nasal Spray from November 29, 2003 to April 6,
2004 is not covered by excess insurance.
The
Company's claim against Wachovia Insurance Services, Inc. and First Union
Insurance Services Agency, Inc. is for negligence and for equitable insurance
for these claims based on the Company's undertaking of certain attorneys' fees
and costs of settlement for claims that should have been covered by underlying
insurance placed by Wachovia Insurance Services, Inc.
At this
time no prediction can be made as to the outcome of any action against Wachovia
Insurance Services, Inc. and First Union Insurance Services Agency,
Inc.